Developing Therapeutics for PrP Prion Diseases
- PMID: 28096242
- PMCID: PMC5378016
- DOI: 10.1101/cshperspect.a023747
Developing Therapeutics for PrP Prion Diseases
Abstract
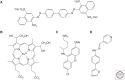
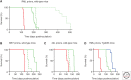
The prototypical PrP prion diseases are invariably fatal, and the search for agents to treat them spans more than 30 years, with limited success. However, in the last few years, the application of high-throughput screening, medicinal chemistry, and pharmacokinetic optimization has led to important advances. The PrP prion inoculation paradigm provides a robust assay for testing therapeutic efficacy, and a dozen compounds have been reported that lead to meaningful extension in survival of prion-infected mice. Here, we review the history and recent progress in the field, focusing on studies validated in animal models. Based on screens in cells infected with mouse-passaged PrP prions, orally available compounds were generated that double or even triple the survival of mice infected with the same prion strain. Unfortunately, no compounds have yet shown efficacy against human prions. Nevertheless, the speed of the recent advances brings hope that an effective therapeutic can be developed. A successful treatment for any neurodegenerative disease would be a major achievement, and the growing understanding that the more common neurodegenerative diseases, including Alzheimer's and Parkinson's, progress by an analogous prion mechanism serves to highlight the importance of antiprion therapeutics.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

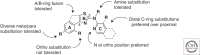



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
