FGF21 Mediates the Thermogenic and Insulin-Sensitizing Effects of Dietary Methionine Restriction but Not Its Effects on Hepatic Lipid Metabolism
- PMID: 28096260
- PMCID: PMC5360300
- DOI: 10.2337/db16-1212
FGF21 Mediates the Thermogenic and Insulin-Sensitizing Effects of Dietary Methionine Restriction but Not Its Effects on Hepatic Lipid Metabolism
Abstract
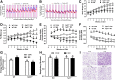
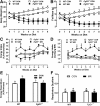
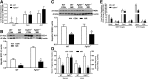
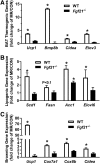
Dietary methionine restriction (MR) produces a rapid and persistent remodeling of white adipose tissue (WAT), an increase in energy expenditure (EE), and enhancement of insulin sensitivity. Recent work established that hepatic expression of FGF21 is robustly increased by MR. Fgf21-/- mice were used to test whether FGF21 is an essential mediator of the physiological effects of dietary MR. The MR-induced increase in energy intake and EE and activation of thermogenesis in WAT and brown adipose tissue were lost in Fgf21-/- mice. However, dietary MR produced a comparable reduction in body weight and adiposity in both genotypes because of a negative effect of MR on energy intake in Fgf21-/- mice. Despite the similar loss in weight, dietary MR produced a more significant increase in in vivo insulin sensitivity in wild-type than in Fgf21-/- mice, particularly in heart and inguinal WAT. In contrast, the ability of MR to regulate lipogenic and integrated stress response genes in liver was not compromised in Fgf21-/- mice. Collectively, these findings illustrate that FGF21 is a critical mediator of the effects of dietary MR on EE, remodeling of WAT, and increased insulin sensitivity but not of its effects on hepatic gene expression.
© 2017 by the American Diabetes Association.
Figures

References
-
- Malloy VL, Krajcik RA, Bailey SJ, Hristopoulos G, Plummer JD, Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell 2006;5:305–314 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

