Tuning Collective Cell Migration by Cell-Cell Junction Regulation
- PMID: 28096261
- PMCID: PMC5378050
- DOI: 10.1101/cshperspect.a029199
Tuning Collective Cell Migration by Cell-Cell Junction Regulation
Abstract
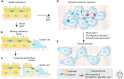
Collective cell migration critically depends on cell-cell interactions coupled to a dynamic actin cytoskeleton. Important cell-cell adhesion receptor systems implicated in controlling collective movements include cadherins, immunoglobulin superfamily members (L1CAM, NCAM, ALCAM), Ephrin/Eph receptors, Slit/Robo, connexins and integrins, and an adaptive array of intracellular adapter and signaling proteins. Depending on molecular composition and signaling context, cell-cell junctions adapt their shape and stability, and this gradual junction plasticity enables different types of collective cell movements such as epithelial sheet and cluster migration, branching morphogenesis and sprouting, collective network migration, as well as coordinated individual-cell migration and streaming. Thereby, plasticity of cell-cell junction composition and turnover defines the type of collective movements in epithelial, mesenchymal, neuronal, and immune cells, and defines migration coordination, anchorage, and cell dissociation. We here review cell-cell adhesion systems and their functions in different types of collective cell migration as key regulators of collective plasticity.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Alexander S, Koehl GE, Hirschberg M, Geissler EK, Friedl P. 2008. Dynamic imaging of cancer growth and invasion: A modified skin-fold chamber model. Histochem Cell Biol 130: 1147–1154. - PubMed
-
- Arvanitis DN, Behar A, Tryoen-Toth P, Bush JO, Jungas T, Vitale N, Davy A. 2013. Ephrin B1 maintains apical adhesion of neural progenitors. Development 140: 2082–2092. - PubMed
-
- Bailey CH, Kaang BK, Chen M, Martin KC, Lim CS, Casadio A, Kandel ER. 1997. Mutation in the phosphorylation sites of MAP kinase blocks learning-related internalization of apCAM in Aplysia sensory neurons. Neuron 18: 913–924. - PubMed
-
- Balda MS, Matter K. 2016. Tight junctions as regulators of tissue remodelling. Curr Opin Cell Biol 42: 94–101. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
