Anti-Factor B and Anti-C3b Autoantibodies in C3 Glomerulopathy and Ig-Associated Membranoproliferative GN
- PMID: 28096309
- PMCID: PMC5407719
- DOI: 10.1681/ASN.2016030343
Anti-Factor B and Anti-C3b Autoantibodies in C3 Glomerulopathy and Ig-Associated Membranoproliferative GN
Abstract
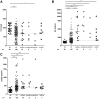
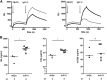
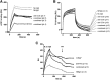
In C3 glomerulopathy (C3G), the alternative pathway of complement is frequently overactivated by autoantibodies that stabilize the C3 convertase C3bBb. Anti-C3b and anti-factor B (anti-FB) IgG have been reported in three patients with C3G. We screened a cohort of 141 patients with C3G and Ig-associated membranoproliferative GN (Ig-MPGN) for anti-FB and anti-C3b autoantibodies using ELISA. We identified seven patients with anti-FB IgG, three patients with anti-C3b IgG, and five patients with anti-FB and anti-C3b IgG. Of these 15 patients, ten were diagnosed with Ig-MPGN. Among those patients with available data, 92% had a nephrotic syndrome, 64% had AKI, and 67% had a documented infection. Patients negative for anti-C3b and anti-FB IgG had much lower rates of infection (17 [25%] patients with C3G and one [10%] patient with Ig-MPGN). After 48 months, four of 15 (26%) positive patients had developed ESRD or died. All 15 patients had high plasma Bb levels, six (40%) patients had low levels of C3, and nine (60%) patients had high levels of soluble C5b9. In vitro, IgG purified from patients with anti-FB Abs selectively enhanced C3 convertase activity; IgG from patients with anti-C3b/anti-FB Abs enhanced C3 and C5 cleavage. IgG from patients with anti-C3b Abs stabilized C3bBb and perturbed C3b binding to complement receptor 1 but did not perturb binding to factor H. In conclusion, the prevalence of anti-C3b/anti-FB Abs and alternative pathway activation is similar in Ig-MPGN and C3G, suggesting similar pathogenic mechanisms. Identification of the underlying defect in Ig-MPGN could lead to improved treatment.
Keywords: chronic glomerulonephritis; clinical immunology; complement.
Copyright © 2017 by the American Society of Nephrology.
Figures





References
-
- Zipfel PF, Skerka C, Chen Q, Wiech T, Goodship T, Johnson S, Fremeaux-Bacchi V, Nester C, de Córdoba SR, Noris M, Pickering M, Smith R: The role of complement in C3 glomerulopathy. Mol Immunol 67: 21–30, 2015 - PubMed
-
- Servais A, Noël LH, Roumenina LT, Le Quintrec M, Ngo S, Dragon-Durey MA, Macher MA, Zuber J, Karras A, Provot F, Moulin B, Grünfeld JP, Niaudet P, Lesavre P, Frémeaux-Bacchi V: Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int 82: 454–464, 2012 - PubMed
-
- Cook HT, Pickering MC: Histopathology of MPGN and C3 glomerulopathies. Nat Rev Nephrol 11: 14–22, 2015 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

