Biophysical properties of the clinical-stage antibody landscape
- PMID: 28096333
- PMCID: PMC5293111
- DOI: 10.1073/pnas.1616408114
Biophysical properties of the clinical-stage antibody landscape
Abstract
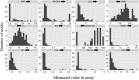
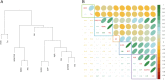
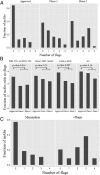
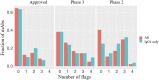
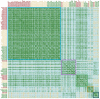
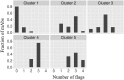
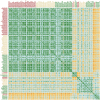
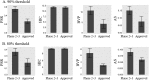
Antibodies are a highly successful class of biological drugs, with over 50 such molecules approved for therapeutic use and hundreds more currently in clinical development. Improvements in technology for the discovery and optimization of high-potency antibodies have greatly increased the chances for finding binding molecules with desired biological properties; however, achieving drug-like properties at the same time is an additional requirement that is receiving increased attention. In this work, we attempt to quantify the historical limits of acceptability for multiple biophysical metrics of "developability." Amino acid sequences from 137 antibodies in advanced clinical stages, including 48 approved for therapeutic use, were collected and used to construct isotype-matched IgG1 antibodies, which were then expressed in mammalian cells. The resulting material for each source antibody was evaluated in a dozen biophysical property assays. The distributions of the observed metrics are used to empirically define boundaries of drug-like behavior that can represent practical guidelines for future antibody drug candidates.
Keywords: biophysical properties; developability; manufacturability; monoclonal antibody; nonspecificity.
Conflict of interest statement
All of the authors are employed by Adimab, LLC, whose business is the discovery of antibody drugs.
Figures

Comment in
-
Non-animal-derived antibodies: pharma companies respond.Nature. 2020 Oct;586(7830):500. doi: 10.1038/d41586-020-02923-z. Nature. 2020. PMID: 33082540 No abstract available.
References
-
- Jacobs SA, Wu SJ, Feng Y, Bethea D, O’Neil KT. Cross-interaction chromatography: A rapid method to identify highly soluble monoclonal antibody candidates. Pharm Res. 2010;27(1):65–71. - PubMed
-
- Nishi H, et al. Fc domain mediated self-association of an IgG1 monoclonal antibody under a low ionic strength condition. J Biosci Bioeng. 2011;112(4):326–332. - PubMed
-
- Lauer TM, et al. Developability index: A rapid in silico tool for the screening of antibody aggregation propensity. J Pharm Sci. 2012;101(1):102–115. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

