Pathogen-mediated manipulation of arthropod microbiota to promote infection
- PMID: 28096373
- PMCID: PMC5293115
- DOI: 10.1073/pnas.1613422114
Pathogen-mediated manipulation of arthropod microbiota to promote infection
Abstract
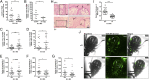
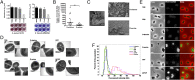
Arthropods transmit diverse infectious agents; however, the ways microbes influence their vector to enhance colonization are poorly understood. Ixodes scapularis ticks harbor numerous human pathogens, including Anaplasma phagocytophilum, the agent of human granulocytic anaplasmosis. We now demonstrate that A. phagocytophilum modifies the I. scapularis microbiota to more efficiently infect the tick. A. phagocytophilum induces ticks to express Ixodes scapularis antifreeze glycoprotein (iafgp), which encodes a protein with several properties, including the ability to alter bacterial biofilm formation. IAFGP thereby perturbs the tick gut microbiota, which influences the integrity of the peritrophic matrix and gut barrier-critical obstacles for Anaplasma colonization. Mechanistically, IAFGP binds the terminal d-alanine residue of the pentapeptide chain of bacterial peptidoglycan, resulting in altered permeability and the capacity of bacteria to form biofilms. These data elucidate the molecular mechanisms by which a human pathogen appropriates an arthropod antibacterial protein to alter the gut microbiota and more effectively colonize the vector.
Keywords: Anaplasma; Ixodes scapularis; antifreeze protein; biofilm; microbiome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Goddard J. Infectious Diseases and Arthropods. Humana; Totowa, NJ: 2000.
-
- Hill CA, Kafatos FC, Stansfield SK, Collins FH. Arthropod-borne diseases: Vector control in the genomics era. Nat Rev Microbiol. 2005;3(3):262–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

