Vimentin intermediate filaments control actin stress fiber assembly through GEF-H1 and RhoA
- PMID: 28096473
- PMCID: PMC5358333
- DOI: 10.1242/jcs.196881
Vimentin intermediate filaments control actin stress fiber assembly through GEF-H1 and RhoA
Abstract
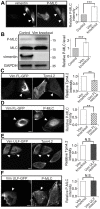
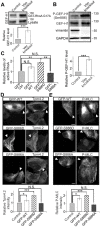
The actin and intermediate filament cytoskeletons contribute to numerous cellular processes, including morphogenesis, cytokinesis and migration. These two cytoskeletal systems associate with each other, but the underlying mechanisms of this interaction are incompletely understood. Here, we show that inactivation of vimentin leads to increased actin stress fiber assembly and contractility, and consequent elevation of myosin light chain phosphorylation and stabilization of tropomyosin-4.2 (see Geeves et al., 2015). The vimentin-knockout phenotypes can be rescued by re-expression of wild-type vimentin, but not by the non-filamentous 'unit length form' vimentin, demonstrating that intact vimentin intermediate filaments are required to facilitate the effects on the actin cytoskeleton. Finally, we provide evidence that the effects of vimentin on stress fibers are mediated by activation of RhoA through its guanine nucleotide exchange factor GEF-H1 (also known as ARHGEF2). Vimentin depletion induces phosphorylation of the microtubule-associated GEF-H1 on Ser886, and thereby promotes RhoA activity and actin stress fiber assembly. Taken together, these data reveal a new mechanism by which intermediate filaments regulate contractile actomyosin bundles, and may explain why elevated vimentin expression levels correlate with increased migration and invasion of cancer cells.
Keywords: Actin; GEF-H1; Intermediate filament; RhoA; Stress fiber; Vimentin.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

