Role of preemptive tapentadol in reduction of postoperative analgesic requirements after laparoscopic cholecystectomy
- PMID: 28096581
- PMCID: PMC5187615
- DOI: 10.4103/0970-9185.168257
Role of preemptive tapentadol in reduction of postoperative analgesic requirements after laparoscopic cholecystectomy
Abstract
Background and aims: Poorly managed acute postoperative pain may result in prolonged morbidity. Various pharmacotherapies have targeted this, but research on an ideal preemptive analgesic continues, taking into account drug-related side effects. Considering the better tolerability profile of tapentadol, we assessed its role as a preemptive analgesic in the reduction of postoperative analgesic requirements, after laparoscopic cholecystectomy.
Material and methods: In a prospective-double-blinded fashion, sixty patients posted for above surgery, were randomized to receive tablet tapentadol 75 mg (Group A) or starch tablets (Group B) orally, an hour before induction of general anesthesia. Perioperative analgesic requirement, time to first analgesia, pain, and sedation score were compared for first 24 h during the postoperative period and analyzed by one-way analysis of variance test. A P < 0.05 was considered significant.
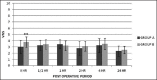
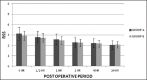
Results: Sixty patients were analyzed. The perioperative analgesic requirement was significantly lower in Group A. Verbal numerical score was significantly lower in Group A at the time point, immediately after shifting the patient to the postanesthesia care unit. Ramsay sedation scores were similar between the groups. No major side effects were observed except for nausea and vomiting in 26 cases (10 in Group A, 16 in Group B).
Conclusion: Single preemptive oral dose of tapentadol (75 mg) is effective in reducing perioperative analgesic requirements and acute postoperative pain, without added side effects. It could be an appropriate preemptive analgesic, subjected to future trials concentrating upon its dose-response effects.
Keywords: Analgesia; cholecystectomy; tapentadol.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–40. - PubMed
-
- Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: Results from a US national survey. Curr Med Res Opin. 2014;30:149–60. - PubMed
-
- Hutchison RW. Challenges in acute post-operative pain management. Am J Health Syst Pharm. 2007;64(6 Suppl 4):S2–5. - PubMed
-
- Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: A meta-analysis. Anesth Analg. 2005;100:757–73. - PubMed
-
- Sandhu T, Paiboonworachat S, Ko-iam W. Effects of preemptive analgesia in laparoscopic cholecystectomy: A double-blind randomized controlled trial. Surg Endosc. 2011;25:23–7. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

