Immune-Checkpoint Inhibitors in the Era of Precision Medicine: What Radiologists Should Know
- PMID: 28096717
- PMCID: PMC5240494
- DOI: 10.3348/kjr.2017.18.1.42
Immune-Checkpoint Inhibitors in the Era of Precision Medicine: What Radiologists Should Know
Abstract
Over the past five years immune-checkpoint inhibitors have dramatically changed the therapeutic landscape of advanced solid and hematologic malignancies. The currently approved immune-checkpoint inhibitors include antibodies to cytotoxic T-lymphocyte antigen-4, programmed cell death (PD-1), and programmed cell death ligand (PD-L1 and PD-L2). Response to immune-checkpoint inhibitors is evaluated on imaging using the immune-related response criteria. Activation of immune system results in a unique toxicity profile termed immune-related adverse events. This article will review the molecular mechanism, clinical applications, imaging of immune-related response patterns and adverse events associated with immune-checkpoint inhibitors.
Keywords: Immune-checkpoint inhibitor; Immune-related Response Criteria; Response; Toxicity.
Conflict of interest statement
Disclosures: Dr. Nishino’s disclosures: Consulting or Advisory Role: Bristol-Myers Squibb, Toshiba Medical Systems, Worldcare Clinical Research Funding: Merck (Inst), Canon (Inst) Dr. Hodi’s disclosures: Consulting or Advisory Role: Merck Sharp & Dohme, Novartis, Genentech, Amgen Research Funding: Bristol-Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Genentech (Inst), Novartis (Inst) Patents, Royalties, Other Intellectual Property: Patent pending as per institutional policy; patent pending royalties received on MICA related disorders application to institution per institutional intellectual property policy Travel, Accommodations, Expenses: Novartis, Bristol-Myers Squibb Other Relationship: Bristol-Myers Squibb, Genentech
Figures
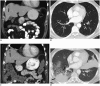
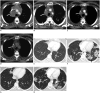

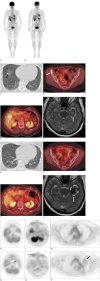
References
-
- Rosenberg SA. Adoptive immunotherapy of cancer: accomplishments and prospects. Cancer Treat Rep. 1984;68:233–255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

