Myocardial T1 and T2 Mapping: Techniques and Clinical Applications
- PMID: 28096723
- PMCID: PMC5240500
- DOI: 10.3348/kjr.2017.18.1.113
Myocardial T1 and T2 Mapping: Techniques and Clinical Applications
Abstract
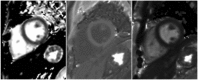
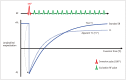
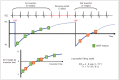
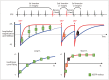
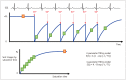
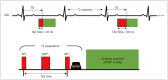
Cardiac magnetic resonance (CMR) imaging is widely used in various medical fields related to cardiovascular diseases. Rapid technological innovations in magnetic resonance imaging in recent times have resulted in the development of new techniques for CMR imaging. T1 and T2 image mapping sequences enable the direct quantification of T1, T2, and extracellular volume fraction (ECV) values of the myocardium, leading to the progressive integration of these sequences into routine CMR settings. Currently, T1, T2, and ECV values are being recognized as not only robust biomarkers for diagnosis of cardiomyopathies, but also predictive factors for treatment monitoring and prognosis. In this study, we have reviewed various T1 and T2 mapping sequence techniques and their clinical applications.
Keywords: Cardiomyopathy; Extracellular matrix; Heart; MRI, T1 mapping; Myocardium; Native T1; T2 mapping.
Figures

References
-
- Radunski UK, Lund GK, Stehning C, Schnackenburg B, Bohnen S, Adam G, et al. CMR in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc Imaging. 2014;7:667–675. - PubMed
-
- Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

