miR-29a Promotes Lipid Droplet and Triglyceride Formation in HCV Infection by Inducing Expression of SREBP-1c and CAV1
- PMID: 28097097
- PMCID: PMC5225148
- DOI: 10.14218/JCTH.2016.00046
miR-29a Promotes Lipid Droplet and Triglyceride Formation in HCV Infection by Inducing Expression of SREBP-1c and CAV1
Abstract
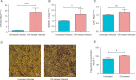
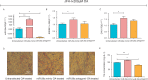
Aims: To examine the regulation of SREBP-1c and CAV1 by microRNA-29a (miR-29a) in cells infected with hepatitis C virus (HCV) in an attempt to control HCV-induced non-alcoholic fatty liver disease. Methods: In order to examine the manipulation of SREBP-1c and CAV1 by miR-29a, oleic acid (OA)-treated JFH-I-infected Huh-7 cells were used. OA was added 24 h post-transfection and gene expression was investigated by qRT-PCR at 48 h post treatment. The functional impact of the observed alteration in SREBP-1c and CAV1 expression was analyzed by examining lipid droplet (LD) and triglyceride (TG) content at 72 h post-OA treatment using light microscopy and spectrophotometry, respectively. Viral load was quantified by qRT-PCR at 72 h post-transfection. Results: OA treatment induced the expression of miR-29a and SREBP-1c, as compared to untreated cells. Forced miR-29a expression led to a significant up-regulation of SREBP-1c as well as CAV1 compared to mock untransfected cells. Ectopic expression of miR-29a resulted in a marked increase in LDs and their respective TGs, while miR-29a antagomirs decreased both the LD and TG content compared to mock untransfected cells. Moreover, forcing the expression of miR-29a in JFH-1 HCV-infected Huh-7 cells resulted in 53% reduction in viral titers compared to mock untransfected Huh-7 cells. Conclusion: Inducing miR-29a expression significantly induces SREBP-1c and CAV1 expression, thereby increasing LDs as well as their respective TGs. Nonetheless, forcing the expression of miR-29a resulted in reduction of HCV RNA levels in Huh-7 cells.
Keywords: Caveolin-1; HCV; Lipid droplets; MicroRNA-29a; SREBP-1c.
Conflict of interest statement
None
Figures


References
-
- Asselah T, Rubbia-Brandt L, Marcellin P, Negro F. Steatosis in chronic hepatitis C: why does it really matter? Gut. 2006;55:123–130. doi: 10.1136/gut.2005.069757. - DOI - PMC - PubMed
-
- Thomopoulos KC, Arvaniti V, Tsamantas AC, Dimitropoulou D, Gogos CA, Siagris D, et al. Prevalence of liver steatosis in patients with chronic hepatitis B: a study of associated factors and of relationship with fibrosis. Eur J Gastroenterol Hepatol. 2006;18:233–237. - PubMed
-
- Mirandola S, Bowman D, Hussain MM, Alberti A. Hepatic steatosis in hepatitis C is a storage disease due to HCV interaction with microsomal triglyceride transfer protein (MTP) Nutr Metab (Lond) 2010;7:13. doi: 10.1186/1743-7075-7-13. - DOI - PMC - PubMed
-
- Singaravelu R, Chen R, Lyn RK, Jones DM, O’Hara S, Rouleau Y, et al. Hepatitis C virus induced up-regulation of microRNA-27: a novel mechanism for hepatic steatosis. Hepatology. 2014;59:98–108. doi: 10.1002/hep.26634. - DOI - PubMed
-
- Afzal MS, Zaidi NU, Dubuisson J, Rouille Y. Hepatitis C virus capsid protein and intracellular lipids interplay and its association with hepatic steatosis. Hepat Mon. 2014;14:e17812. doi: 10.5812/hepatmon.17812. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
