Inhibitor of Nicotinamide Phosphoribosyltransferase Sensitizes Glioblastoma Cells to Temozolomide via Activating ROS/JNK Signaling Pathway
- PMID: 28097126
- PMCID: PMC5206411
- DOI: 10.1155/2016/1450843
Inhibitor of Nicotinamide Phosphoribosyltransferase Sensitizes Glioblastoma Cells to Temozolomide via Activating ROS/JNK Signaling Pathway
Abstract
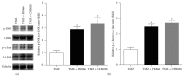
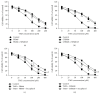
Overcoming temozolomide (TMZ) resistance is a great challenge in glioblastoma (GBM) treatment. Nicotinamide phosphoribosyltransferase (NAMPT) is a rate-limiting enzyme in the biosynthesis of nicotinamide adenine dinucleotide and has a crucial role in cancer cell metabolism. In this study, we investigated whether FK866 and CHS828, two specific NAMPT inhibitors, could sensitize GBM cells to TMZ. Low doses of FK866 and CHS828 (5 nM and 10 nM, resp.) alone did not significantly decrease cell viability in U251-MG and T98 GBM cells. However, they significantly increased the antitumor action of TMZ in these cells. In U251-MG cells, administration of NAMPT inhibitors increased the TMZ (100 μM)-induced apoptosis and LDH release from GBM cells. NAMPT inhibitors remarkably enhanced the activities of caspase-1, caspase-3, and caspase-9. Moreover, NAMPT inhibitors increased reactive oxygen species (ROS) production and superoxide anion level but reduced the SOD activity and total antioxidative capacity in GBM cells. Treatment of NAMPT inhibitors increased phosphorylation of c-Jun and JNK. Administration of JNK inhibitor SP600125 or ROS scavenger tocopherol with TMZ and NAMPT inhibitors substantially attenuated the sensitization of NAMPT inhibitor on TMZ antitumor action. Our data indicate a potential value of NAMPT inhibitors in combined use with TMZ for GBM treatment.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

