Lung vaso-occlusion in sickle cell disease mediated by arteriolar neutrophil-platelet microemboli
- PMID: 28097236
- PMCID: PMC5214368
- DOI: 10.1172/jci.insight.89761
Lung vaso-occlusion in sickle cell disease mediated by arteriolar neutrophil-platelet microemboli
Abstract
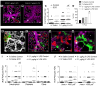
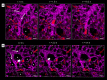
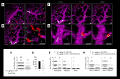
In patients with sickle cell disease (SCD), the polymerization of intraerythrocytic hemoglobin S promotes downstream vaso-occlusive events in the microvasculature. While vaso-occlusion is known to occur in the lung, often in the context of systemic vaso-occlusive crisis and the acute chest syndrome, the pathophysiological mechanisms that incite lung injury are unknown. We used intravital microscopy of the lung in transgenic humanized SCD mice to monitor acute vaso-occlusive events following an acute dose of systemic lipopolysaccharide sufficient to trigger events in SCD but not control mice. We observed cellular microembolism of precapillary pulmonary arteriolar bottlenecks by neutrophil-platelet aggregates. Blood from SCD patients was next studied under flow in an in vitro microfluidic system. Similar to the pulmonary circulation, circulating platelets nucleated around arrested neutrophils, translating to a greater number and duration of neutrophil-platelet interactions compared with normal human blood. Inhibition of platelet P-selectin with function-blocking antibody attenuated the neutrophil-platelet interactions in SCD patient blood in vitro and resolved pulmonary arteriole microembolism in SCD mice in vivo. These results establish the relevance of neutrophil-platelet aggregate formation in lung arterioles in promoting lung vaso-occlusion in SCD and highlight the therapeutic potential of targeting platelet adhesion molecules to prevent acute chest syndrome.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures




References
-
- GBD 2013 Mortality Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

