Fc-Silent Anti-CD154 Domain Antibody Effectively Prevents Nonhuman Primate Renal Allograft Rejection
- PMID: 28097811
- PMCID: PMC5409881
- DOI: 10.1111/ajt.14197
Fc-Silent Anti-CD154 Domain Antibody Effectively Prevents Nonhuman Primate Renal Allograft Rejection
Abstract
The advent of costimulation blockade provides the prospect for targeted therapy with improved graft survival in transplant patients. Perhaps the most effective costimulation blockade in experimental models is the use of reagents to block the CD40/CD154 pathway. Unfortunately, successful clinical translation of anti-CD154 therapy has not been achieved. In an attempt to develop an agent that is as effective as previous CD154 blocking antibodies but lacks the risk of thromboembolism, we evaluated the efficacy and safety of a novel anti-human CD154 domain antibody (dAb, BMS-986004). The anti-CD154 dAb effectively blocked CD40-CD154 interactions but lacked crystallizable fragment (Fc) binding activity and resultant platelet activation. In a nonhuman primate kidney transplant model, anti-CD154 dAb was safe and efficacious, significantly prolonging allograft survival without evidence of thromboembolism (Median survival time 103 days). The combination of anti-CD154 dAb and conventional immunosuppression synergized to effectively control allograft rejection (Median survival time 397 days). Furthermore, anti-CD154 dAb treatment increased the frequency of CD4+ CD25+ Foxp3+ regulatory T cells. This study demonstrates that the use of a novel anti-CD154 dAb that lacks Fc binding activity is safe without evidence of thromboembolism and is equally as potent as previous anti-CD154 agents at prolonging renal allograft survival in a nonhuman primate preclinical model.
Keywords: animal models: nonhuman primate; basic (laboratory) research/science; costimulation; fusion proteins and monoclonal antibodies: costimulation molecule specific; immunosuppressant; immunosuppression/immune modulation; rejection: T cell mediated (TCMR); translational research/science.
© 2017 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have conflicts of interest to disclose as described by the
Figures
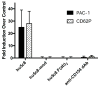
 ) or CD62P (
) or CD62P (
 ) expression.
) expression.

 ), 2mg/kg (n=2) (
), 2mg/kg (n=2) (
 )). (B) Animals treated with 20mg/kg maintained good renal function as measured by serum creatinine. Animals treated with lower doses had accelerated rises in their laboratory values consistent with their shortened rejection-free survival times. (C) After the first infusion on the date of transplant, the level of anti- CD154 dAb peaked on post-operative day 1 and subsequently decreased until day 7 when they received their next infusion. The 20mg/kg dose maintained trough levels >20ug/ml.
)). (B) Animals treated with 20mg/kg maintained good renal function as measured by serum creatinine. Animals treated with lower doses had accelerated rises in their laboratory values consistent with their shortened rejection-free survival times. (C) After the first infusion on the date of transplant, the level of anti- CD154 dAb peaked on post-operative day 1 and subsequently decreased until day 7 when they received their next infusion. The 20mg/kg dose maintained trough levels >20ug/ml.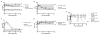
 ,PT/Fibrinogen/D-Dimer/Antithrombin III
,PT/Fibrinogen/D-Dimer/Antithrombin III
 ) were maintained in the normal range. Animals who did not receive anti-CD154 dAb as part of their treatment regimen were also tested as controls (
) were maintained in the normal range. Animals who did not receive anti-CD154 dAb as part of their treatment regimen were also tested as controls (
 ). (E) Platelet counts were maintained above 100 (103/μL) with the 20mg/kg dosing regimen (20mg/kg —, 10mg/kg
). (E) Platelet counts were maintained above 100 (103/μL) with the 20mg/kg dosing regimen (20mg/kg —, 10mg/kg
 , 2mg/kg
, 2mg/kg
 ) and comparable to control animals who did not receive anti-CD154 dAb (
) and comparable to control animals who did not receive anti-CD154 dAb (
 ).
).
 ) (MST 397 vs MST 103). (B) Animals treated with anti-CD154 and conventional immunosuppression had excellent renal function as assessed by serum creatinine (C) CMV viral load was used as a surrogate for overall level of immunosuppression Only one animal in the monotherapy group developed detectable CMV viremia which was quickly controlled with anti-viral therapy. No animal in the combined group developed detectable levels of virus.
) (MST 397 vs MST 103). (B) Animals treated with anti-CD154 and conventional immunosuppression had excellent renal function as assessed by serum creatinine (C) CMV viral load was used as a surrogate for overall level of immunosuppression Only one animal in the monotherapy group developed detectable CMV viremia which was quickly controlled with anti-viral therapy. No animal in the combined group developed detectable levels of virus.

 ) or anti-CD154 dAb + conventional therapy (n=5) (
) or anti-CD154 dAb + conventional therapy (n=5) (
 ). (B) Animals receiving conventional immunosuppression in addition to anti-CD154 dAb demonstrated a significantly lower frequency of terminally differentiated CCR7-CD45RA+ CD4+ T cells during their time on therapy. Graphs depict mean ± standard error of the mean (SEM).
). (B) Animals receiving conventional immunosuppression in addition to anti-CD154 dAb demonstrated a significantly lower frequency of terminally differentiated CCR7-CD45RA+ CD4+ T cells during their time on therapy. Graphs depict mean ± standard error of the mean (SEM).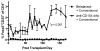
 ), the frequency of CD25+Foxp3+ phenotypically regulatory T cells was increased compared to standard costimulation blockade therapy (n=9) (
), the frequency of CD25+Foxp3+ phenotypically regulatory T cells was increased compared to standard costimulation blockade therapy (n=9) (
 ). Differences between treatment groups were significant for time points over the entire duration of therapy. Graphs depict mean ± SEM.
). Differences between treatment groups were significant for time points over the entire duration of therapy. Graphs depict mean ± SEM.Comment in
-
A Tale of Two Pathways: Renewing the Promise of Anti-CD40L Blockade.Am J Transplant. 2017 May;17(5):1156-1157. doi: 10.1111/ajt.14217. Epub 2017 Feb 28. Am J Transplant. 2017. PMID: 28141921 No abstract available.
Similar articles
-
TNX-1500, a crystallizable fragment-modified anti-CD154 antibody, prolongs nonhuman primate renal allograft survival.Am J Transplant. 2023 Aug;23(8):1171-1181. doi: 10.1016/j.ajt.2023.03.022. Epub 2023 Apr 4. Am J Transplant. 2023. PMID: 37019335 Free PMC article.
-
A novel, blocking, Fc-silent anti-CD40 monoclonal antibody prolongs nonhuman primate renal allograft survival in the absence of B cell depletion.Am J Transplant. 2015 Nov;15(11):2825-36. doi: 10.1111/ajt.13377. Epub 2015 Jul 2. Am J Transplant. 2015. PMID: 26139432
-
CD154 blockade effectively controls antibody-mediated rejection in highly sensitized nonhuman primate kidney transplant recipients.Sci Transl Med. 2025 Jan;17(779):eadn8130. doi: 10.1126/scitranslmed.adn8130. Epub 2025 Jan 1. Sci Transl Med. 2025. PMID: 39742504 Free PMC article.
-
The Inhibition of CD40/CD154 Costimulatory Signaling in the Prevention of Renal Transplant Rejection in Nonhuman Primates: A Systematic Review and Meta Analysis.Front Immunol. 2022 Apr 7;13:861471. doi: 10.3389/fimmu.2022.861471. eCollection 2022. Front Immunol. 2022. PMID: 35464470 Free PMC article.
-
Novel insights into anti-CD40/CD154 immunotherapy in transplant tolerance.Immunotherapy. 2015;7(4):399-410. doi: 10.2217/imt.15.1. Immunotherapy. 2015. PMID: 25917630 Free PMC article. Review.
Cited by
-
Emerging Therapies in Immune Thrombocytopenia.J Clin Med. 2021 Mar 2;10(5):1004. doi: 10.3390/jcm10051004. J Clin Med. 2021. PMID: 33801294 Free PMC article. Review.
-
Implantable niche with local immunosuppression for islet allotransplantation achieves type 1 diabetes reversal in rats.Nat Commun. 2022 Dec 26;13(1):7951. doi: 10.1038/s41467-022-35629-z. Nat Commun. 2022. PMID: 36572684 Free PMC article.
-
Toward Small-Molecule Inhibition of Protein-Protein Interactions: General Aspects and Recent Progress in Targeting Costimulatory and Coinhibitory (Immune Checkpoint) Interactions.Curr Top Med Chem. 2018;18(8):674-699. doi: 10.2174/1568026618666180531092503. Curr Top Med Chem. 2018. PMID: 29848279 Free PMC article. Review.
-
Design, Synthesis, and Evaluation of Novel Immunomodulatory Small Molecules Targeting the CD40⁻CD154 Costimulatory Protein-Protein Interaction.Molecules. 2018 May 11;23(5):1153. doi: 10.3390/molecules23051153. Molecules. 2018. PMID: 29751636 Free PMC article.
-
The Role of Costimulation Blockade in Solid Organ and Islet Xenotransplantation.J Immunol Res. 2017;2017:8415205. doi: 10.1155/2017/8415205. Epub 2017 Oct 11. J Immunol Res. 2017. PMID: 29159187 Free PMC article. Review.
References
-
- Larsen C, Elwood E, DZ A, SC R, RH, CT-B, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature Atlanta. 1996;381(6581):434–8. - PubMed
-
- Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5(3):443–53. - PubMed
-
- Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med [Internet] 1999 Jun;5(6):686–93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10371508. - PubMed
-
- Pearson TC, Trambley J, Odom K, Anderson DC, Cowan SR, Bray R, et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation [Internet] 2002;74(7):933–40. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id.... - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

