Human farnesyl pyrophosphate synthase is allosterically inhibited by its own product
- PMID: 28098152
- PMCID: PMC5253651
- DOI: 10.1038/ncomms14132
Human farnesyl pyrophosphate synthase is allosterically inhibited by its own product
Abstract
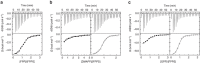
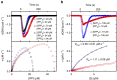
Farnesyl pyrophosphate synthase (FPPS) is an enzyme of the mevalonate pathway and a well-established therapeutic target. Recent research has focused around a newly identified druggable pocket near the enzyme's active site. Pharmacological exploitation of this pocket is deemed promising; however, its natural biological function, if any, is yet unknown. Here we report that the product of FPPS, farnesyl pyrophosphate (FPP), can bind to this pocket and lock the enzyme in an inactive state. The Kd for this binding is 5-6 μM, within a catalytically relevant range. These results indicate that FPPS activity is sensitive to the product concentration. Kinetic analysis shows that the enzyme is inhibited through FPP accumulation. Having a specific physiological effector, FPPS is a bona fide allosteric enzyme. This allostery offers an exquisite mechanism for controlling prenyl pyrophosphate levels in vivo and thus contributes an additional layer of regulation to the mevalonate pathway.
Figures


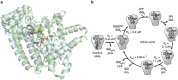
References
-
- Hosfield D. J. et al. Structural basis for bisphosphonate-mediated inhibition of isoprenoid biosynthesis. J. Biol. Chem. 279, 8526–8529 (2004). - PubMed
-
- Rondeau J. M. et al. Structural basis for the exceptional in vivo efficacy of bisphosphonate drugs. ChemMedChem. 1, 267–273 (2006). - PubMed
-
- Russell R. G. Bisphosphonates: the first 40 years. Bone 49, 2–19 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

