IFN-γ regulates human dental pulp stem cells behavior via NF-κB and MAPK signaling
- PMID: 28098169
- PMCID: PMC5241669
- DOI: 10.1038/srep40681
IFN-γ regulates human dental pulp stem cells behavior via NF-κB and MAPK signaling
Abstract
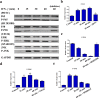
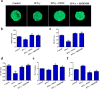
During caries, dental pulp expresses a range of pro-inflammatory cytokines in response to the infectious challenge. Interferon gamma (IFN-γ) is a dimerized soluble cytokine, which is critical for immune responses. Previous study has demonstrated that IFN-γ at relative high concentration (100 ng/mL) treatment improved the impaired dentinogenic and immunosuppressive regulatory functions of disease-derived dental pulp stem cells (DPSCs). However, little is known about the regulatory effects of IFN-γ at relative low concentration on healthy DPSC behavior (including proliferation, migration, and multiple-potential differentiation). Here we demonstrate that IFN-γ at relatively low concentrations (0.5 ng/mL) promoted the proliferation and migration of DPSCs, but abrogated odonto/osteogenic differentiation. Additionally, we identified that NF-κB and MAPK signaling pathways are both involved in the process of IFN-γ-regulated odonto/osteogenic differentiation of DPSCs. DPSCs treated with IFN-γ and supplemented with pyrrolidine dithiocarbamate (PDTC, an NF-κB inhibitor) or SB203580 (a MAPK inhibitor) showed significantly improved potential for odonto/osteogenic differentiation of DPSCs both in vivo and in vitro. These data provide important insight into the regulatory effects of IFN-γ on the biological behavior of DPSCs and indicate a promising therapeutic strategy for dentin/pulp tissue engineering in future endodontic treatment.
Figures

Similar articles
-
Interferon-gamma improves impaired dentinogenic and immunosuppressive functions of irreversible pulpitis-derived human dental pulp stem cells.Sci Rep. 2016 Jan 18;6:19286. doi: 10.1038/srep19286. Sci Rep. 2016. PMID: 26775677 Free PMC article.
-
Estrogen deficiency inhibits the odonto/osteogenic differentiation of dental pulp stem cells via activation of the NF-κB pathway.Cell Tissue Res. 2013 Jun;352(3):551-9. doi: 10.1007/s00441-013-1604-z. Epub 2013 Mar 27. Cell Tissue Res. 2013. PMID: 23532562
-
Interferon Gamma-treated Dental Pulp Stem Cells Promote Human Mesenchymal Stem Cell Migration In Vitro.J Endod. 2015 Aug;41(8):1259-64. doi: 10.1016/j.joen.2015.02.018. Epub 2015 Jun 4. J Endod. 2015. PMID: 26051078 Free PMC article.
-
Mechanical Signaling in Dental Pulp Stem Cells.Front Biosci (Landmark Ed). 2023 Oct 31;28(10):274. doi: 10.31083/j.fbl2810274. Front Biosci (Landmark Ed). 2023. PMID: 37919075 Review.
-
Epigenetic modulation of dental pulp stem cells: implications for regenerative endodontics.Int Endod J. 2016 May;49(5):431-46. doi: 10.1111/iej.12475. Epub 2015 Jun 11. Int Endod J. 2016. PMID: 26011759 Review.
Cited by
-
MicroRNA-506 Is Involved in Regulation of the Occurrence of Lipopolysaccharides (LPS)-Induced Pulpitis by Sirtuin 1 (SIRT1).Med Sci Monit. 2019 Dec 26;25:10008-10015. doi: 10.12659/MSM.918172. Med Sci Monit. 2019. PMID: 31877121 Free PMC article.
-
Amphiregulin regulates odontogenic differentiation of dental pulp stem cells by activation of mitogen-activated protein kinase and the phosphatidylinositol 3-kinase signaling pathways.Stem Cell Res Ther. 2022 Jul 15;13(1):304. doi: 10.1186/s13287-022-02971-4. Stem Cell Res Ther. 2022. PMID: 35841013 Free PMC article.
-
Application of Biocompatible Scaffolds in Stem-Cell-Based Dental Tissue Engineering.Adv Exp Med Biol. 2023;1409:83-110. doi: 10.1007/5584_2022_734. Adv Exp Med Biol. 2023. PMID: 35999347 Review.
-
Guanylate-Binding Protein 1 Promotes Migration and Invasion of Human Periodontal Ligament Stem Cells.Stem Cells Int. 2018 Nov 28;2018:6082956. doi: 10.1155/2018/6082956. eCollection 2018. Stem Cells Int. 2018. PMID: 30622567 Free PMC article.
-
Effects of different signaling pathways on odontogenic differentiation of dental pulp stem cells: a review.Front Physiol. 2023 Oct 19;14:1272764. doi: 10.3389/fphys.2023.1272764. eCollection 2023. Front Physiol. 2023. PMID: 37929208 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

