Hepatitis B virus X protein is capable of down-regulating protein level of host antiviral protein APOBEC3G
- PMID: 28098260
- PMCID: PMC5241686
- DOI: 10.1038/srep40783
Hepatitis B virus X protein is capable of down-regulating protein level of host antiviral protein APOBEC3G
Abstract
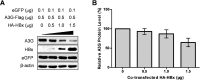
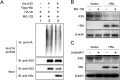
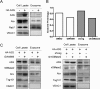
The apolipoprotein B mRNA editing catalytic polypeptide-like (APOBEC) family proteins bind RNA and single-stranded DNA, and create C-to-U base modifications through cytidine deaminase activity. APOBEC3G restricts human immunodeficiency virus 1 (HIV-1) infection by creating hypermutations in proviral DNA, while HIV-1-encoded vif protein antagonizes such restriction by targeting APOBEC3G for degradation. APOBEC3G also inhibits hepatitis B virus (HBV): APOBEC3G co-expression inhibits HBV replication and evidences exist indicating APOBEC3G-mediated HBV hypermutations in patients. HBV encodes a small non-structural X protein (HBx) with a recognized activating effect on HBV life cycle. In this work, we report the discovery that HBx selectively and dose-dependently decreases the protein level of co-expressed APOBEC3G in transfected Huh-7 cells. The effect was shown to take place post-translationally, but does not rely on protein degradation via proteasome or lysosome. Further work demonstrated that intracellular APOBEC3G is normally exported via exosome secretion and inhibition of exosome biogenesis causes retention of intracellular APOBEC3G. Finally, HBx co-expression specifically enhanced externalization of APOBEC3G via exosomes, resulting in decrease of intracellular APOBEC3G protein level. These data suggest the possibility that in addition to other mechanisms, HBx-mediated activation of HBV might also involve antagonizing of intracellular restriction factor APOBEC3G through promotion of its export.
Figures



References
-
- World Health Organization. Hepatitis B. World Health Organization Fact Sheet 204 (Revised July 2016). http://www.who.int/mediacentre/factsheets/fs204/en/ (2016).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

