Mechano-chemo-transduction in cardiac myocytes
- PMID: 28098356
- PMCID: PMC5471413
- DOI: 10.1113/JP273101
Mechano-chemo-transduction in cardiac myocytes
Abstract
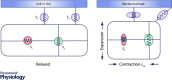
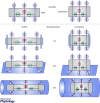
The heart has the ability to adjust to changing mechanical loads. The Frank-Starling law and the Anrep effect describe exquisite intrinsic mechanisms the heart has for autoregulating the force of contraction to maintain cardiac output under changes of preload and afterload. Although these mechanisms have been known for more than a century, their cellular and molecular underpinnings are still debated. How does the cardiac myocyte sense changes in preload or afterload? How does the myocyte adjust its response to compensate for such changes? In cardiac myocytes Ca2+ is a crucial regulator of contractile force and in this review we compare and contrast recent studies from different labs that address these two important questions. The 'dimensionality' of the mechanical milieu under which experiments are carried out provide important clues to the location of the mechanosensors and the kinds of mechanical forces they can sense and respond to. As a first approximation, sensors inside the myocyte appear to modulate reactive oxygen species while sensors on the cell surface appear to also modulate nitric oxide signalling; both signalling pathways affect Ca2+ handling. Undoubtedly, further studies will add layers to this simplified picture. Clarifying the intimate links from cellular mechanics to reactive oxygen species and nitric oxide signalling and to Ca2+ handling will deepen our understanding of the Frank-Starling law and the Anrep effect, and also provide a unified view on how arrhythmias may arise in seemingly disparate diseases that have in common altered myocyte mechanics.
Keywords: calcium signalling; cardiac arrhythmia; cardiac myocytes; heart disease; mechanotransduction; muscle mechanics; nitric oxide synthase; reactive oxygen species.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures
References
-
- Alvarez BV, Perez NG, Ennis IL, Camilion de Hurtado MC & Cingolani HE (1999). Mechanisms underlying the increase in force and Ca2+ transient that follow stretch of cardiac muscle: a possible explanation of the Anrep effect. Circ Res 85, 716–722. - PubMed
-
- Anastasi G, Cutroneo G, Gaeta R, Di Mauro D, Arco A, Consolo A, Santoro G, Trimarchi F & Favaloro A (2009). Dystrophin‐glycoprotein complex and vinculin‐talin‐integrin system in human adult cardiac muscle. Int J Mol Med 23, 149–159. - PubMed
-
- Awasthi S, Izu L, Mao Z, Jian Z, Landas T, Lerner A, Shimkunas R, Woldeyesus RA, Bossuyt J, Wood BM, Chen Y‐J, Matthews DL, Lieu DK, Chiamvimonvat N, Lam KS, Chen‐Izu Y & Chan JW (2015). Multimodal SHG‐2PF imaging of microdomain Ca2+‐contraction coupling in live cardiac myocytes. Circ Res 118, e19–e28. - PMC - PubMed
-
- Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H & Labeit S (2001). The complete gene sequence of titin, expression of an unusual approximately 700‐kDa titin isoform, and its interaction with obscurin identify a novel Z‐line to I‐band linking system. Circ Res 89, 1065–1072. - PubMed
-
- Berlin JR, Cannell MB & Lederer WJ (1989). Cellular origins of the transient inward current in cardiac myocytes. Role of fluctuations and waves of elevated intracellular calcium. Circ Res 65 115–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

