Comparative Solid-State Stability of Perindopril Active Substance vs. Pharmaceutical Formulation
- PMID: 28098840
- PMCID: PMC5297797
- DOI: 10.3390/ijms18010164
Comparative Solid-State Stability of Perindopril Active Substance vs. Pharmaceutical Formulation
Abstract
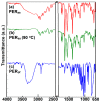
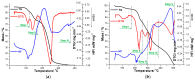
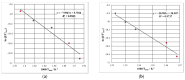
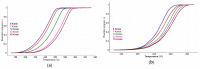
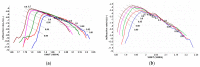
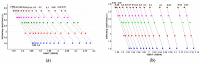
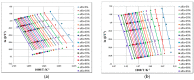
This paper presents the results obtained after studying the thermal stability and decomposition kinetics of perindopril erbumine as a pure active pharmaceutical ingredient as well as a solid pharmaceutical formulation containing the same active pharmaceutical ingredient (API). Since no data were found in the literature regarding the spectroscopic description, thermal behavior, or decomposition kinetics of perindopril, our goal was the evaluation of the compatibility of this antihypertensive agent with the excipients in the tablet under ambient conditions and to study the effect of thermal treatment on the stability of perindopril erbumine. ATR-FTIR (Attenuated Total Reflectance Fourier Transform Infrared) spectroscopy, thermal analysis (thermogravimetric mass curve (TG-thermogravimetry), derivative thermogravimetric mass curve (DTG), and heat flow (HF)) and model-free kinetics were chosen as investigational tools. Since thermal behavior is a simplistic approach in evaluating the thermal stability of pharmaceuticals, in-depth kinetic studies were carried out by classical kinetic methods (Kissinger and ASTM E698) and later with the isoconversional methods of Friedman, Kissinger-Akahira-Sunose and Flynn-Wall-Ozawa. It was shown that the main thermal degradation step of perindopril erbumine is characterized by activation energy between 59 and 69 kJ/mol (depending on the method used), while for the tablet, the values were around 170 kJ/mol. The used excipients (anhydrous colloidal silica, microcrystalline cellulose, lactose, and magnesium stearate) should be used in newly-developed generic solid pharmaceutical formulations, since they contribute to an increased thermal stability of perindopril erbumine.
Keywords: ASTM E698; comparative stability; decomposition; isoconversional kinetic study; perindopril erbumine; perindopril tert-butylamine; pharmaceutical formulation; thermal stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mancia G., Fagard R., Narkiewicz K., Redon J., Zanchetti A., Bohm M., Christiaens T., Cifkova R., de Backer G., Dominiczak A., et al. Task force for the management of arterial hypertension of the European society of hypertension and the European society of cardiology. 2013 ESH/ESC guidelines for the management of arterial hypertension. Eur. Heart J. 2013;34:2159–2219. - PubMed
-
- Fox K. Contribution of perindopril to cardiology: 20 years of success. Eur. Heart J. 2007;9:E10–E19. doi: 10.1093/eurheartj/sum038. - DOI
-
- Tantu M., Belu E., Bobescu E., Armean S.M., Armean P., Constantin M.M., Dominaru C.D. Role of angiotensin converting enzyme (ACE) inhibitors in hypertension and cardiovascular protection management. Farmacia. 2014;62:451–459.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

