Characterization, regulation, and targeting of erythroid progenitors in normal and disordered human erythropoiesis
- PMID: 28099275
- PMCID: PMC5518670
- DOI: 10.1097/MOH.0000000000000328
Characterization, regulation, and targeting of erythroid progenitors in normal and disordered human erythropoiesis
Abstract
Purpose of review: The erythroid progenitors burst-forming unit-erythroid and colony-forming unit-erythroid have a critical role in erythropoiesis. These cells represent a heterogeneous and poorly characterized population with modifiable self-renewal, proliferation and differentiation capabilities. This review focuses on the current state of erythroid progenitor biology with regard to immunophenotypic identification and regulatory programs. In addition, we will discuss the therapeutic implications of using these erythroid progenitors as pharmacologic targets.
Recent findings: Erythroid progenitors are classically characterized by the appearance of morphologically defined colonies in semisolid cultures. However, these prior systems preclude a more thorough understanding of the composite nature of progenitor populations. Recent studies employing novel flow cytometric and cell-based assays have helped to redefine hematopoiesis, and suggest that erythroid progenitors may arise from different levels of the hematopoietic tree. Moreover, the identification of cell surface marker patterns in human burst-forming unit-erythroid and colony-forming unit-erythroid enhance our ability to perform downstream functional and molecular analyses at the population and single cell level. Advances in these techniques have already revealed novel subpopulations with increased self-renewing capacity, roles for erythroid progenitors in globin gene expression, and insights into pharmacologic mechanisms of glucocorticoids and pomalidomide.
Summary: Immunophenotypic and molecular characterization resolves the diversity of erythroid progenitors, and may ultimately lead to the ability to target these progenitors to ameliorate diseases of dyserythropoiesis.
Conflict of interest statement
None.
Figures
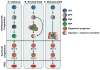

References
-
- Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: a human perspective. Cell Stem Cell. 2012;10(2):120–36. - PubMed
-
- Koury MJ. Abnormal erythropoiesis and the pathophysiology of chronic anemia. Blood Rev. 2014;28(2):49–66. - PubMed
-
- Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121(2):295–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

