Multicenter Study Validating Accuracy of a Continuous Respiratory Rate Measurement Derived From Pulse Oximetry: A Comparison With Capnography
- PMID: 28099286
- PMCID: PMC5367492
- DOI: 10.1213/ANE.0000000000001852
Multicenter Study Validating Accuracy of a Continuous Respiratory Rate Measurement Derived From Pulse Oximetry: A Comparison With Capnography
Abstract
Background: Intermittent measurement of respiratory rate via observation is routine in many patient care settings. This approach has several inherent limitations that diminish the clinical utility of these measurements because it is intermittent, susceptible to human error, and requires clinical resources. As an alternative, a software application that derives continuous respiratory rate measurement from a standard pulse oximeter has been developed. We sought to determine the performance characteristics of this new technology by comparison with clinician-reviewed capnography waveforms in both healthy subjects and hospitalized patients in a low-acuity care setting.
Methods: Two independent observational studies were conducted to validate the performance of the Medtronic Nellcor Respiration Rate Software application. One study enrolled 26 healthy volunteer subjects in a clinical laboratory, and a second multicenter study enrolled 53 hospitalized patients. During a 30-minute study period taking place while participants were breathing spontaneously, pulse oximeter and nasal/oral capnography waveforms were collected. Pulse oximeter waveforms were processed to determine respiratory rate via the Medtronic Nellcor Respiration Rate Software. Capnography waveforms reviewed by a clinician were used to determine the reference respiratory rate.
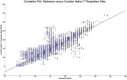
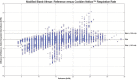
Results: A total of 23,243 paired observations between the pulse oximeter-derived respiratory rate and the capnography reference method were collected and examined. The mean reference-based respiratory rate was 15.3 ± 4.3 breaths per minute with a range of 4 to 34 breaths per minute. The Pearson correlation coefficient between the Medtronic Nellcor Respiration Rate Software values and the capnography reference respiratory rate is reported as a linear correlation, R, as 0.92 ± 0.02 (P < .001), whereas Lin's concordance correlation coefficient indicates an overall agreement of 0.85 ± 0.04 (95% confidence interval [CI] +0.76; +0.93) (healthy volunteers: 0.94 ± 0.02 [95% CI +0.91; +0.97]; hospitalized patients: 0.80 ± 0.06 [95% CI +0.68; +0.92]). The mean bias of the Medtronic Nellcor Respiration Rate Software was 0.18 breaths per minute with a precision (SD) of 1.65 breaths per minute (healthy volunteers: 0.37 ± 0.78 [95% limits of agreement: -1.16; +1.90] breaths per minute; hospitalized patients: 0.07 ± 1.99 [95% limits of agreement: -3.84; +3.97] breaths per minute). The root mean square deviation was 1.35 breaths per minute (healthy volunteers: 0.81; hospitalized patients: 1.60).
Conclusions: These data demonstrate the performance of the Medtronic Nellcor Respiration Rate Software in healthy subjects and patients hospitalized in a low-acuity care setting when compared with clinician-reviewed capnography. The observed performance of this technology suggests that it may be a useful adjunct to continuous pulse oximetry monitoring by providing continuous respiratory rate measurements. The potential patient safety benefit of using combined continuous pulse oximetry and respiratory rate monitoring warrants assessment.
Conflict of interest statement
Conflicts of Interest: See Disclosures at the end of the article.
Figures
Comment in
-
In Response.Anesth Analg. 2017 Sep;125(3):1077-1078. doi: 10.1213/ANE.0000000000002292. Anesth Analg. 2017. PMID: 28708663 No abstract available.
-
Replication of Data Makes Statistical Analysis Difficult.Anesth Analg. 2017 Sep;125(3):1076-1077. doi: 10.1213/ANE.0000000000002293. Anesth Analg. 2017. PMID: 28708667 No abstract available.
-
In Response.Anesth Analg. 2017 Sep;125(3):1075-1076. doi: 10.1213/ANE.0000000000002294. Anesth Analg. 2017. PMID: 28742782 No abstract available.
-
Limits of Agreement With Confidence Intervals Are Necessary to Assess Comparability of Measurement Devices.Anesth Analg. 2017 Sep;125(3):1075. doi: 10.1213/ANE.0000000000002295. Anesth Analg. 2017. PMID: 28759494 No abstract available.
References
-
- Buist M, Bernard S, Nguyen TV, Moore G, Anderson J.Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62:137–141. - PubMed
-
- Barker SJ, Tremper KK, Gamel DM.A clinical comparison of transcutaneous PO2 and pulse oximetry in the operating room. Anesth Analg. 1986;65:805–808. - PubMed
-
- Kodali BS.Capnography outside the operating rooms. Anesthesiology. 2013;118:192–201. - PubMed
-
- Holley K, MacNabb CM, Georgiadis P, Minasyan H, Shukla A, Mathews D.Monitoring minute ventilation versus respiratory rate to measure the adequacy of ventilation in patients undergoing upper endoscopic procedures. J Clin Monit Comput. 2016;30:33–39. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials