IL-17 is a neuromodulator of Caenorhabditis elegans sensory responses
- PMID: 28099418
- PMCID: PMC5503128
- DOI: 10.1038/nature20818
IL-17 is a neuromodulator of Caenorhabditis elegans sensory responses
Abstract
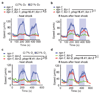
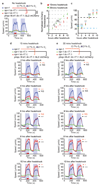
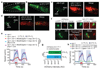
Interleukin-17 (IL-17) is a major pro-inflammatory cytokine: it mediates responses to pathogens or tissue damage, and drives autoimmune diseases. Little is known about its role in the nervous system. Here we show that IL-17 has neuromodulator-like properties in Caenorhabditis elegans. IL-17 can act directly on neurons to alter their response properties and contribution to behaviour. Using unbiased genetic screens, we delineate an IL-17 signalling pathway and show that it acts in the RMG hub interneurons. Disrupting IL-17 signalling reduces RMG responsiveness to input from oxygen sensors, and renders sustained escape from 21% oxygen transient and contingent on additional stimuli. Over-activating IL-17 receptors abnormally heightens responses to 21% oxygen in RMG neurons and whole animals. IL-17 deficiency can be bypassed by optogenetic stimulation of RMG. Inducing IL-17 expression in adults can rescue mutant defects within 6 h. These findings reveal a non-immunological role of IL-17 modulating circuit function and behaviour.
Conflict of interest statement
The authors declare no competing financial interests.
Figures











Comment in
-
Interleukin-17: Why the Worms Squirm.Immunity. 2017 Mar 21;46(3):347-349. doi: 10.1016/j.immuni.2017.03.007. Immunity. 2017. PMID: 28329701
-
Neuromodulation: The Fevered Mind of the Worm.Curr Biol. 2017 Apr 24;27(8):R315-R317. doi: 10.1016/j.cub.2017.03.005. Curr Biol. 2017. PMID: 28441568
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

