Oral Tolerance Induced by OVA Intake Ameliorates TNBS-Induced Colitis in Mice
- PMID: 28099498
- PMCID: PMC5242488
- DOI: 10.1371/journal.pone.0170205
Oral Tolerance Induced by OVA Intake Ameliorates TNBS-Induced Colitis in Mice
Abstract
Introduction: Literature data have shown that the consumption of dietary proteins may cause modulatory effects on the host immune system, process denominated oral tolerance by bystander suppression. It has been shown that the bystander suppression induced by dietary proteins can improve inflammatory diseases such as experimental arthritis. Here, we evaluated the effects of oral tolerance induced by ingestion of ovalbumin (OVA) on TNBS-induced colitis in mice, an experimental model for human Crohn's disease.
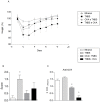
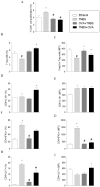
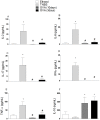
Methods and results: Colitis was induced in BALB/c mice by instilling a single dose of TNBS (100 mg/kg) in ethanol into the colon. Tolerized mice received OVA (4mg/mL) dissolved in the drinking water for seven consecutive days, prior to or concomitantly with the intrarectal instillation. Control groups received protein-free water and ethanol by intrarectal route. We observed that either the prior or concomitant induction of oral tolerance were able to reduce the severity of colitis as noted by recovery of body weight gain, improvement of clinical signs and reduction of histological abnormalities. The in vitro proliferation of spleen cells from tolerant colitic mice was lower than that of control mice, the same as the frequencies of CD4+ T cells secreting IL-17 and IFN-γ. The frequencies of regulatory T cells and T cells secreting IL-10 have increased significantly in mice orally treated with OVA. The levels of inflammatory cytokines (IL-17A, TNF-α, IL-6 and IFN-γ) were lower in supernatants of cells from tolerant colitic mice, whereas IL-10 levels were higher.
Conclusion: Our data show that the modulation of immune response induced by oral tolerance reduces the severity of experimental colitis. Such modulation may be partially attributed to the increase of Treg cells and reduction of pro-inflammatory cytokines in peripheral lymphoid organs of tolerant mice by bystander suppression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Adoptive transfer of dendritic cells expressing CD11c reduces the immunological response associated with experimental colitis in BALB/c mice.PLoS One. 2018 May 8;13(5):e0196994. doi: 10.1371/journal.pone.0196994. eCollection 2018. PLoS One. 2018. PMID: 29738575 Free PMC article.
-
Rectal administration of lipopolysaccharide and ovalbumin ameliorates acute murine colitis.Dig Dis Sci. 2011 Aug;56(8):2292-8. doi: 10.1007/s10620-011-1630-1. Epub 2011 Feb 26. Dig Dis Sci. 2011. PMID: 21359540
-
Amelioration of immune-mediated experimental colitis: tolerance induction in the presence of preexisting immunity and surrogate antigen bystander effect.J Pharmacol Exp Ther. 2001 Jun;297(3):926-32. J Pharmacol Exp Ther. 2001. PMID: 11356912
-
Mycophenolate Mofetil Modulates Differentiation of Th1/Th2 and the Secretion of Cytokines in an Active Crohn's Disease Mouse Model.Int J Mol Sci. 2015 Nov 6;16(11):26654-66. doi: 10.3390/ijms161125985. Int J Mol Sci. 2015. PMID: 26561804 Free PMC article.
-
Induction of ovalbumin-specific tolerance by oral administration of Lactococcus lactis secreting ovalbumin.Gastroenterology. 2007 Aug;133(2):517-28. doi: 10.1053/j.gastro.2007.04.073. Epub 2007 May 3. Gastroenterology. 2007. PMID: 17681173
Cited by
-
Long-Term Oral Administration of Hyperimmune Egg-Based IgY-Rich Formulations Induces Mucosal Immune Response and Systemic Increases of Cytokines Involved in Th2- and Th17-Type Immune Responses in C57BL/6 Mice.Int J Mol Sci. 2024 Aug 9;25(16):8701. doi: 10.3390/ijms25168701. Int J Mol Sci. 2024. PMID: 39201385 Free PMC article.
-
The interplay between the microbiota, diet and T regulatory cells in the preservation of the gut barrier in inflammatory bowel disease.Front Microbiol. 2023 Dec 1;14:1291724. doi: 10.3389/fmicb.2023.1291724. eCollection 2023. Front Microbiol. 2023. PMID: 38107848 Free PMC article. Review.
-
Adoptive transfer of dendritic cells expressing CD11c reduces the immunological response associated with experimental colitis in BALB/c mice.PLoS One. 2018 May 8;13(5):e0196994. doi: 10.1371/journal.pone.0196994. eCollection 2018. PLoS One. 2018. PMID: 29738575 Free PMC article.
-
RANKL Triggers Treg-Mediated Immunoregulation in Inflammatory Osteolysis.J Dent Res. 2018 Jul;97(8):917-927. doi: 10.1177/0022034518759302. Epub 2018 Mar 2. J Dent Res. 2018. PMID: 29499125 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

