3D Functional Corneal Stromal Tissue Equivalent Based on Corneal Stromal Stem Cells and Multi-Layered Silk Film Architecture
- PMID: 28099503
- PMCID: PMC5242458
- DOI: 10.1371/journal.pone.0169504
3D Functional Corneal Stromal Tissue Equivalent Based on Corneal Stromal Stem Cells and Multi-Layered Silk Film Architecture
Abstract
The worldwide need for human cornea equivalents continues to grow. Few clinical options are limited to allogenic and synthetic material replacements. We hypothesized that tissue engineered human cornea systems based on mechanically robust, patterned, porous, thin, optically clear silk protein films, in combination with human corneal stromal stem cells (hCSSCs), would generate 3D functional corneal stroma tissue equivalents, in comparison to previously developed 2D approaches. Silk film contact guidance was used to control the alignment and distribution of hCSSCs on RGD-treated single porous silk films, which were then stacked in an orthogonally, multi-layered architecture and cultured for 9 weeks. These systems were compared similar systems generated with human corneal fibroblasts (hCFs). Both cell types were viable and preferentially aligned along the biomaterial patterns for up to 9 weeks in culture. H&E histological sections showed that the systems seeded with the hCSSCs displayed ECM production throughout the entire thickness of the constructs. In addition, the ECM proteins tested positive for keratocyte-specific tissue markers, including keratan sulfate, lumican, and keratocan. The quantification of hCSSC gene expression of keratocyte-tissue markers, including keratocan, lumican, human aldehyde dehydrogenase 3A1 (ALDH3A1), prostaglandin D2 synthase (PTDGS), and pyruvate dehydrogenase kinase, isozyme 4 (PDK4), within the 3D tissue systems demonstrated upregulation when compared to 2D single silk films and to the systems generated with the hCFs. Furthermore, the production of ECM from the hCSSC seeded systems and subsequent remodeling of the initial matrix significantly improved cohesiveness and mechanical performance of the constructs, while maintaining transparency after 9 weeks.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

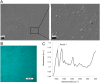

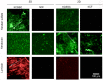


References
-
- Jacobs J, Taravella M. Corneal graft rejection. eMedicine. 2005(1):1–3.
-
- 2013 Eye Banking Statistical Report. http://www.restoresight.org/wp-content/uploads/2014/04/2013_Statistical_... Eye Bank Association of America; 2013 Jan 22, 2015.
-
- Griffith M, Harkin DG. Recent advances in the design of artificial corneas. Current opinion in ophthalmology. 2014;25(3):240–7. - PubMed
-
- Ronge L. LASIK shatters assumptions. Cornea. 1994(13):379–82.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

