Clinical Practice Guidelines for Rare Diseases: The Orphanet Database
- PMID: 28099516
- PMCID: PMC5242437
- DOI: 10.1371/journal.pone.0170365
Clinical Practice Guidelines for Rare Diseases: The Orphanet Database
Abstract
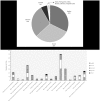
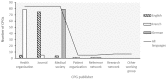
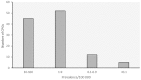
Clinical practice guidelines (CPGs) for rare diseases (RDs) are scarce, may be difficult to identify through Internet searches and may vary in quality depending on the source and methodology used. In order to contribute to the improvement of the diagnosis, treatment and care of patients, Orphanet (www.orpha.net) has set up a procedure for the selection, quality evaluation and dissemination of CPGs, with the aim to provide easy access to relevant, accurate and specific recommendations for the management of RDs. This article provides an analysis of selected CPGs by medical domain coverage, prevalence of diseases, languages and type of producer, and addresses the variability in CPG quality and availability. CPGs are identified via bibliographic databases, websites of research networks, expert centres or medical societies. They are assessed according to quality criteria derived from the Appraisal of Guidelines, REsearch and Evaluation (AGREE II) Instrument. Only open access CPGs and documents for which permission from the copyright holders has been obtained are disseminated on the Orphanet website. From January 2012 to July 2015, 277 CPGs were disseminated, representing coverage of 1,122 groups of diseases, diseases or subtypes in the Orphanet database. No language restriction is applied, and so far 10 languages are represented, with a predominance of CPGs in English, French and German (92% of all CPGs). A large proportion of diseases with identified CPGs belong to rare oncologic, neurologic, hematologic diseases or developmental anomalies. The Orphanet project on CPG collection, evaluation and dissemination is a continuous process, with regular addition of new guidelines, and updates. CPGs meeting the quality criteria are integrated to the Orphanet database of rare diseases, together with other types of textual information and the appropriate services for patients, researchers and healthcare professionals in 40 countries.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Field MJ and Lohr KN (eds.). Clinical practice guidelines: directions for a new program, Institute of Medicine. National Academy Press, Washington, DC: 1990. - PubMed
-
- Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on orphan medicinal products. http://eur-lex.europa.eu/legal-content/CS/ALL/?uri=URISERV:l21167. Accessed 10 Dec 2015.
-
- Kirschner J, Rodger S, Vry J, Gramsch K, Lochmüller H, Bushby K. How reference networks develop, implement, and monitor guidelines. Orphanet J Rare Dis. 2012; 7(Suppl 2): A14–A14.
-
- Sejersen T, Del Giovane C, Filippini G, Leo CG, Meerpohl JJ, Mincarone P, et al. Methodology for production of best practice guidelines for rare diseases. Rare Dis Orphan Drugs. 2014; 1(1):10–19.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

