Nanomolar-Potency Aminophenyl-1,3,5-triazine Activators of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Chloride Channel for Prosecretory Therapy of Dry Eye Diseases
- PMID: 28099811
- PMCID: PMC5453640
- DOI: 10.1021/acs.jmedchem.6b01792
Nanomolar-Potency Aminophenyl-1,3,5-triazine Activators of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Chloride Channel for Prosecretory Therapy of Dry Eye Diseases
Abstract
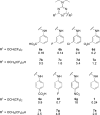
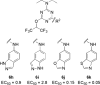
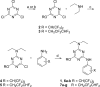
Dry eye disorders are a significant health problem for which limited therapeutic options are available. CFTR is a major prosecretory chloride channel at the ocular surface. We previously identified, by high-throughput screening, aminophenyl-1,3,5-triazine CFTRact-K089 (1) that activated CFTR with EC50 ≈ 250 nM, which when delivered topically increased tear fluid secretion in mice and showed efficacy in an experimental dry eye model. Here, functional analysis of aminophenyl-1,3,5-triazine analogs elucidated structure-activity relationships for CFTR activation and identified substantially more potent analogs than 1. The most potent compound, 12, fully activated CFTR chloride conductance with EC50 ≈ 30 nM, without causing cAMP or calcium elevation. 12 was rapidly metabolized by hepatic microsomes, which supports its topical use. Single topical administration of 25 pmol of 12 increased tear volume in wild-type mice with sustained action for 8 h and was without effect in CFTR-deficient mice. Topically delivered 12 may be efficacious in human dry eye diseases.
Conflict of interest statement
The authors declare the following competing financial interest(s): Drs. Levin and Verkman are named co-inventors on a provisional patent application on CFTR activators for dry eye therapy filed December 2015, whose rights are owned by the University of California.
Figures







Similar articles
-
Small-molecule CFTR activators increase tear secretion and prevent experimental dry eye disease.FASEB J. 2016 May;30(5):1789-97. doi: 10.1096/fj.201500180. Epub 2016 Feb 3. FASEB J. 2016. PMID: 26842854 Free PMC article.
-
Nanomolar Potency Aminophenyltriazine CFTR Activator Reverses Corneal Epithelial Injury in a Mouse Model of Dry Eye.J Ocul Pharmacol Ther. 2020 Apr;36(3):147-153. doi: 10.1089/jop.2019.0087. Epub 2020 Jan 14. J Ocul Pharmacol Ther. 2020. PMID: 31934802 Free PMC article.
-
Pro-Secretory Activity and Pharmacology in Rabbits of an Aminophenyl-1,3,5-Triazine CFTR Activator for Dry Eye Disorders.Invest Ophthalmol Vis Sci. 2017 Sep 1;58(11):4506-4513. doi: 10.1167/iovs.17-22525. Invest Ophthalmol Vis Sci. 2017. PMID: 28873176 Free PMC article.
-
CFTR chloride channel drug discovery--inhibitors as antidiarrheals and activators for therapy of cystic fibrosis.Curr Pharm Des. 2006;12(18):2235-47. doi: 10.2174/138161206777585148. Curr Pharm Des. 2006. PMID: 16787252 Review.
-
Cystic fibrosis transmembrane conductance regulator and the outwardly rectifying chloride channel: a relationship between two chloride channels expressed in epithelial cells.Clin Exp Pharmacol Physiol. 2000 Nov;27(11):892-5. doi: 10.1046/j.1440-1681.2000.03356.x. Clin Exp Pharmacol Physiol. 2000. PMID: 11071305 Review.
Cited by
-
Ocular Surface Ion Transport and Dry Eye Disease.Curr Ophthalmol Rep. 2022 Dec;10(4):188-197. doi: 10.1007/s40135-022-00295-3. Epub 2022 Oct 20. Curr Ophthalmol Rep. 2022. PMID: 38213468 Free PMC article.
-
Ocular Surface Potential Difference Measured in Human Subjects to Study Ocular Surface Ion Transport.Transl Vis Sci Technol. 2020 Oct 15;9(11):20. doi: 10.1167/tvst.9.11.20. eCollection 2020 Oct. Transl Vis Sci Technol. 2020. PMID: 33117611 Free PMC article.
-
Novel Insight Into the Role of CFTR in Lacrimal Gland Duct Function in Mice.Invest Ophthalmol Vis Sci. 2018 Jan 1;59(1):54-62. doi: 10.1167/iovs.17-22533. Invest Ophthalmol Vis Sci. 2018. PMID: 29305607 Free PMC article.
-
Calcium-sensing receptor (CaSR) modulates ocular surface chloride transport and its inhibition promotes ocular surface hydration.Ocul Surf. 2024 Oct;34:30-37. doi: 10.1016/j.jtos.2024.06.002. Epub 2024 Jun 11. Ocul Surf. 2024. PMID: 38871216
-
Overview of CFTR activators and their recent studies for dry eye disease: a review.RSC Med Chem. 2023 Sep 25;14(12):2459-2472. doi: 10.1039/d3md00448a. eCollection 2023 Dec 13. RSC Med Chem. 2023. PMID: 38107177 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical

