Replication Study: Discovery and preclinical validation of drug indications using compendia of public gene expression data
- PMID: 28100397
- PMCID: PMC5245962
- DOI: 10.7554/eLife.17044
Replication Study: Discovery and preclinical validation of drug indications using compendia of public gene expression data
Abstract
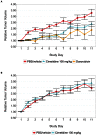
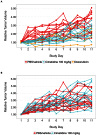
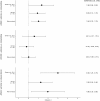
In 2015, as part of the Reproducibility Project: Cancer Biology, we published a Registered Report (Kandela et al., 2015) that described how we intended to replicate selected experiments from the paper "Discovery and Preclinical Validation of Drug Indications Using Compendia of Public Gene Expression Data" (Sirota et al., 2011). Here we report the results of those experiments. We found that cimetidine treatment in a xenograft model using A549 lung adenocarcinoma cells resulted in decreased tumor volume compared to vehicle control; however, while the effect was in the same direction as the original study (Figure 4C; Sirota et al., 2011), it was not statistically significant. Cimetidine treatment in a xenograft model using ACHN renal cell carcinoma cells did not differ from vehicle control treatment, similar to the original study (Supplemental Figure 1; Sirota et al., 2011). Doxorubicin treatment in a xenograft model using A549 lung adenocarcinoma cells did not result in a statistically significant difference compared to vehicle control despite tumor volume being reduced to levels similar to those reported in the original study (Figure 4C; Sirota et al., 2011). Finally, we report a random effects meta-analysis for each result. These meta-analyses show that the inhibition of A549 derived tumors by cimetidine resulted in a statistically significant effect, as did the inhibition of A549 derived tumors by doxorubicin. The effect of cimetidine on ACHN derived tumors was not statistically significant, as predicted.
Keywords: Reproducibility Project: Cancer Biology; cancer biology; cimetidine; drug retargeting; human; metascience; replication; reproducibility.
Conflict of interest statement
IK and FA: Developmental Therapeutics Core is a Science Exchange associated lab. RP:CB: EI, NP: Employed by and hold shares in Science Exchange Inc.
Figures

Comment in
-
Mixed outcomes for computational predictions.Elife. 2017 Jan 19;6:e22661. doi: 10.7554/eLife.22661. Elife. 2017. PMID: 28100399 Free PMC article.
References
-
- Hossain MA, Kim DH, Jang JY, Kang YJ, Yoon JH, Moon JO, Chung HY, Kim GY, Choi YH, Copple BL, Kim ND. Aspirin enhances doxorubicin-induced apoptosis and reduces tumor growth in human hepatocellular carcinoma cells in vitro and in vivo. International Journal of Oncology. 2012;40:1636–1642. doi: 10.3892/ijo.2012.1359. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

