The Randomized, Controlled Trial of Late Surfactant: Effects on Respiratory Outcomes at 1-Year Corrected Age
- PMID: 28100402
- PMCID: PMC5367937
- DOI: 10.1016/j.jpeds.2016.12.059
The Randomized, Controlled Trial of Late Surfactant: Effects on Respiratory Outcomes at 1-Year Corrected Age
Abstract
Objective: To determine the effects of late surfactant on respiratory outcomes determined at 1-year corrected age in the Trial of Late Surfactant (TOLSURF), which randomized newborns of extremely low gestational age (≤28 weeks' gestational age) ventilated at 7-14 days to late surfactant and inhaled nitric oxide vs inhaled nitric oxide-alone (control).
Study design: Caregivers were surveyed in a double-blinded manner at 3, 6, 9, and 12 months' corrected age to collect information on respiratory resource use (infant medication use, home support, and hospitalization). Infants were classified for composite outcomes of pulmonary morbidity (no PM, determined in infants with no reported respiratory resource use) and persistent PM (determined in infants with any resource use in ≥3 surveys).
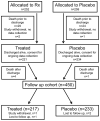
Results: Infants (n = 450, late surfactant n = 217, control n = 233) were 25.3 ± 1.2 weeks' gestation and 713 ± 164 g at birth. In the late surfactant group, fewer infants received home respiratory support than in the control group (35.8% vs 52.9%, relative benefit [RB] 1.28 [95% CI 1.07-1.55]). There was no benefit of late surfactant for No PM vs PM (RB 1.27; 95% CI 0.89-1.81) or no persistent PM vs persistent PM (RB 1.01; 95% CI 0.87-1.17). After adjustment for imbalances in baseline characteristics, relative benefit of late surfactant treatment increased: RB 1.40 (95% CI 0.89-1.80) for no PM and RB 1.24 (95% CI 1.08-1.42) for no persistent PM.
Conclusion: Treatment of newborns of extremely low gestational age with late surfactant in combination with inhaled nitric oxide decreased use of home respiratory support and may decrease persistent pulmonary morbidity.
Trial registration: ClinicalTrials.gov: NCT01022580.
Keywords: bronchopulmonary dysplasia; prematurity; pulmonary morbidity; wheeze.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The other authors declare no conflicts of interest.
Figures
Comment in
-
Is preventing BPD the target, or should we strive for something else?J Pediatr. 2017 Apr;183:2. doi: 10.1016/j.jpeds.2017.02.016. J Pediatr. 2017. PMID: 28343533 No abstract available.
-
Reply.J Pediatr. 2017 Oct;189:241-242. doi: 10.1016/j.jpeds.2017.05.064. Epub 2017 Jun 16. J Pediatr. 2017. PMID: 28625499 No abstract available.
-
Short-, medium-, and long-term assessment of pulmonary dysfunction in extremely low birth weight neonates.J Pediatr. 2017 Oct;189:240-241. doi: 10.1016/j.jpeds.2017.05.062. Epub 2017 Jun 16. J Pediatr. 2017. PMID: 28625500 No abstract available.
References
-
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–60. - PubMed
-
- Watson RS, Clermont G, Kinsella JP, Kong L, Arendt RE, Cutter G, et al. Clinical and economic effects of iNO in premature newborns with respiratory failure at 1 year. Pediatrics. 2009;124:1333–43. - PubMed
-
- Johnson AH, Peacock JL, Greenough A, Marlow N, Limb ES, Marston L, et al. High-frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. N Engl J Med. 2002;347:633–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

