High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial
- PMID: 28100438
- PMCID: PMC5159618
- DOI: 10.1016/S1473-3099(16)30274-2
High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial
Abstract
Background: Tuberculosis is the world's leading infectious disease killer. We aimed to identify shorter, safer drug regimens for the treatment of tuberculosis.
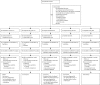
Methods: We did a randomised controlled, open-label trial with a multi-arm, multi-stage design. The trial was done in seven sites in South Africa and Tanzania, including hospitals, health centres, and clinical trial centres. Patients with newly diagnosed, rifampicin-sensitive, previously untreated pulmonary tuberculosis were randomly assigned in a 1:1:1:1:2 ratio to receive (all orally) either 35 mg/kg rifampicin per day with 15-20 mg/kg ethambutol, 20 mg/kg rifampicin per day with 400 mg moxifloxacin, 20 mg/kg rifampicin per day with 300 mg SQ109, 10 mg/kg rifampicin per day with 300 mg SQ109, or a daily standard control regimen (10 mg/kg rifampicin, 5 mg/kg isoniazid, 25 mg/kg pyrazinamide, and 15-20 mg/kg ethambutol). Experimental treatments were given with oral 5 mg/kg isoniazid and 25 mg/kg pyrazinamide per day for 12 weeks, followed by 14 weeks of 5 mg/kg isoniazid and 10 mg/kg rifampicin per day. Because of the orange discoloration of body fluids with higher doses of rifampicin it was not possible to mask patients and clinicians to treatment allocation. The primary endpoint was time to culture conversion in liquid media within 12 weeks. Patients without evidence of rifampicin resistance on phenotypic test who took at least one dose of study treatment and had one positive culture on liquid or solid media before or within the first 2 weeks of treatment were included in the primary analysis (modified intention to treat). Time-to-event data were analysed using a Cox proportional-hazards regression model and adjusted for minimisation variables. The proportional hazard assumption was tested using Schoelfeld residuals, with threshold p<0·05 for non-proportionality. The trial is registered with ClinicalTrials.gov (NCT01785186).
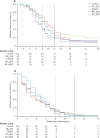
Findings: Between May 7, 2013, and March 25, 2014, we enrolled and randomly assigned 365 patients to different treatment arms (63 to rifampicin 35 mg/kg, isoniazid, pyrazinamide, and ethambutol; 59 to rifampicin 10 mg/kg, isoniazid, pyrazinamide, SQ109; 57 to rifampicin 20 mg/kg, isoniazid, pyrazinamide, and SQ109; 63 to rifampicin 10 mg/kg, isoniazid, pyrazinamide, and moxifloxacin; and 123 to the control arm). Recruitment was stopped early in the arms containing SQ109 since prespecified efficacy thresholds were not met at the planned interim analysis. Time to stable culture conversion in liquid media was faster in the 35 mg/kg rifampicin group than in the control group (median 48 days vs 62 days, adjusted hazard ratio 1·78; 95% CI 1·22-2·58, p=0·003), but not in other experimental arms. There was no difference in any of the groups in time to culture conversion on solid media. 11 patients had treatment failure or recurrent disease during post-treatment follow-up: one in the 35 mg/kg rifampicin arm and none in the moxifloxacin arm. 45 (12%) of 365 patients reported grade 3-5 adverse events, with similar proportions in each arm.
Interpretation: A dose of 35 mg/kg rifampicin was safe, reduced the time to culture conversion in liquid media, and could be a promising component of future, shorter regimens. Our adaptive trial design was successfully implemented in a multi-centre, high tuberculosis burden setting, and could speed regimen development at reduced cost.
Funding: The study was funded by the European and Developing Countries Clinical Trials partnership (EDCTP), the German Ministry for Education and Research (BmBF), and the Medical Research Council UK (MRC).
Copyright © 2017 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Finding the right dose of rifampicin, and the right dose of optimism.Lancet Infect Dis. 2017 Jan;17(1):2-3. doi: 10.1016/S1473-3099(16)30315-2. Epub 2016 Oct 26. Lancet Infect Dis. 2017. PMID: 28100437 No abstract available.
References
-
- Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999;3:S231–S279. - PubMed
-
- van Ingen J, Aarnoutse RE, Donald PR. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin Infect Dis. 2011;52:e194–e199. - PubMed
-
- de Steenwinkel JE, Aarnoutse RE, de Knegt GJ. Optimization of the rifampin dosage to improve the therapeutic efficacy in tuberculosis treatment using a murine model. Am J Respir Crit Care Med. 2013;187:1127–1134. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

