Kinetic analyses of vasculogenesis inform mechanistic studies
- PMID: 28100488
- PMCID: PMC5407022
- DOI: 10.1152/ajpcell.00367.2016
Kinetic analyses of vasculogenesis inform mechanistic studies
Abstract
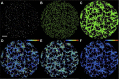
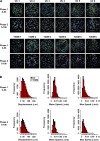
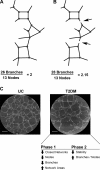
Vasculogenesis is a complex process by which endothelial stem and progenitor cells undergo de novo vessel formation. Quantitative assessment of vasculogenesis is a central readout of endothelial progenitor cell functionality. However, current assays lack kinetic measurements. To address this issue, new approaches were developed to quantitatively assess in vitro endothelial colony-forming cell (ECFC) network formation in real time. Eight parameters of network structure were quantified using novel Kinetic Analysis of Vasculogenesis (KAV) software. KAV assessment of structure complexity identified two phases of network formation. This observation guided the development of additional vasculogenic readouts. A tissue cytometry approach was established to quantify the frequency and localization of dividing ECFCs. Additionally, Fiji TrackMate was used to quantify ECFC displacement and speed at the single-cell level during network formation. These novel approaches were then implemented to identify how intrauterine exposure to maternal diabetes mellitus (DM) impairs fetal ECFC vasculogenesis. Fetal ECFCs exposed to maternal DM form fewer initial network structures, which are not stable over time. Correlation analyses demonstrated that ECFC samples with greater division in branches form fewer closed network structures. Additionally, reductions in average ECFC movement over time decrease structural connectivity. Identification of these novel phenotypes utilizing the newly established methodologies provides evidence for the cellular mechanisms contributing to aberrant ECFC vasculogenesis.
Keywords: diabetes; endothelial; migration; proliferation; vasculogenesis.
Copyright © 2017 the American Physiological Society.
Figures



Similar articles
-
Transgelin induces dysfunction of fetal endothelial colony-forming cells from gestational diabetic pregnancies.Am J Physiol Cell Physiol. 2018 Oct 1;315(4):C502-C515. doi: 10.1152/ajpcell.00137.2018. Epub 2018 Jun 27. Am J Physiol Cell Physiol. 2018. PMID: 29949406 Free PMC article.
-
Kinetic Analysis of Vasculogenesis Quantifies Dynamics of Vasculogenesis and Angiogenesis In Vitro.J Vis Exp. 2018 Jan 31;(131):57044. doi: 10.3791/57044. J Vis Exp. 2018. PMID: 29443032 Free PMC article.
-
Human platelet lysate improves human cord blood derived ECFC survival and vasculogenesis in three dimensional (3D) collagen matrices.Microvasc Res. 2015 Sep;101:72-81. doi: 10.1016/j.mvr.2015.06.006. Epub 2015 Jun 27. Microvasc Res. 2015. PMID: 26122935 Free PMC article.
-
Endothelial progenitor cells for vasculogenesis.Physiology (Bethesda). 2005 Feb;20:36-42. doi: 10.1152/physiol.00033.2004. Physiology (Bethesda). 2005. PMID: 15653838 Review.
-
Proteases and receptors in the recruitment of endothelial progenitor cells in neovascularization.Eur Cytokine Netw. 2009 Dec;20(4):207-19. doi: 10.1684/ecn.2009.0174. Eur Cytokine Netw. 2009. PMID: 20167560 Review.
Cited by
-
Integrated Cytometry With Machine Learning Applied to High-Content Imaging of Human Kidney Tissue for In Situ Cell Classification and Neighborhood Analysis.Lab Invest. 2023 Jun;103(6):100104. doi: 10.1016/j.labinv.2023.100104. Epub 2023 Feb 4. Lab Invest. 2023. PMID: 36867975 Free PMC article.
-
Engineering bioactive nanoparticles to rejuvenate vascular progenitor cells.Commun Biol. 2022 Jun 29;5(1):635. doi: 10.1038/s42003-022-03578-4. Commun Biol. 2022. PMID: 35768543 Free PMC article.
-
Transgelin induces dysfunction of fetal endothelial colony-forming cells from gestational diabetic pregnancies.Am J Physiol Cell Physiol. 2018 Oct 1;315(4):C502-C515. doi: 10.1152/ajpcell.00137.2018. Epub 2018 Jun 27. Am J Physiol Cell Physiol. 2018. PMID: 29949406 Free PMC article.
-
Deep Semantic Segmentation of Angiogenesis Images.Int J Mol Sci. 2023 Jan 6;24(2):1102. doi: 10.3390/ijms24021102. Int J Mol Sci. 2023. PMID: 36674617 Free PMC article.
-
Kinetic Analysis of Vasculogenesis Quantifies Dynamics of Vasculogenesis and Angiogenesis In Vitro.J Vis Exp. 2018 Jan 31;(131):57044. doi: 10.3791/57044. J Vis Exp. 2018. PMID: 29443032 Free PMC article.
References
-
- Abcam Anti-alpha tubulin antibody (DM1A) [Online]. http://www.abcam.com/alpha-tubulin-antibody-dm1a-loading-control-ab7291..... [2017].
-
- Allier CP, Kesavan SV, Coutard JG, Cioni O, Momey F, Navarro F, Menneteau M, Chalmond B, Obeid P, Haguet V, David-Watine B, Dubrulle N, Shorte S, van der Sanden B, Di Natale C, Hamard L, Wion D, Dolega ME, Picollet-D'hahan N, Gidrol X, Dinten JM. Video lensfree microscopy of 2D and 3D cultures of cells. In: Proceedings of SPIE, Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues XII, edited by Farkas DL, Nicolau DV, and Leif RC. San Francisco, CA: SPIE, 2014, vol. 8947, p. 89471H. doi:10.1117/12.2038098. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

