Accuracy of line probe assays for the diagnosis of pulmonary and multidrug-resistant tuberculosis: a systematic review and meta-analysis
- PMID: 28100546
- PMCID: PMC5898952
- DOI: 10.1183/13993003.01075-2016
Accuracy of line probe assays for the diagnosis of pulmonary and multidrug-resistant tuberculosis: a systematic review and meta-analysis
Abstract
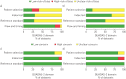
Only 25% of multidrug-resistant tuberculosis (MDR-TB) cases are currently diagnosed. Line probe assays (LPAs) enable rapid drug-susceptibility testing for rifampicin (RIF) and isoniazid (INH) resistance and Mycobacterium tuberculosis detection. Genotype MTBDRplusV1 was WHO-endorsed in 2008 but newer LPAs have since been developed.This systematic review evaluated three LPAs: Hain Genotype MTBDRplusV1, MTBDRplusV2 and Nipro NTM+MDRTB. Study quality was assessed with QUADAS-2. Bivariate random-effects meta-analyses were performed for direct and indirect testing. Results for RIF and INH resistance were compared to phenotypic and composite (incorporating sequencing) reference standards. M. tuberculosis detection results were compared to culture.74 unique studies were included. For RIF resistance (21 225 samples), pooled sensitivity and specificity (with 95% confidence intervals) were 96.7% (95.6-97.5%) and 98.8% (98.2-99.2%). For INH resistance (20 954 samples), pooled sensitivity and specificity were 90.2% (88.2-91.9%) and 99.2% (98.7-99.5%). Results were similar for direct and indirect testing and across LPAs. Using a composite reference standard, specificity increased marginally. For M. tuberculosis detection (3451 samples), pooled sensitivity was 94% (89.4-99.4%) for smear-positive specimens and 44% (20.2-71.7%) for smear-negative specimens.In patients with pulmonary TB, LPAs have high sensitivity and specificity for RIF resistance and high specificity and good sensitivity for INH resistance. This meta-analysis provides evidence for policy and practice.
Copyright ©ERS 2017.
Conflict of interest statement
Conflict of interest: None declared.
Figures






References
-
- World Health Organization. Global Tuberculosis Report. Geneva, World Health Organization, 2016. Available from: http://www.who.int/tb/publications/global_report/en/
-
- World Health Organization. Multidrug and Extensively Drug-resistant TB (M/XDR-TB): 2010 Global Report on Surveillance and Response. Geneva, World Health Organization, 2010. Available from: http://apps.who.int/iris/bitstream/10665/44286/1/9789241599191_eng.pdf
-
- Heifets LB, Cangelosi GA. Drug susceptibility testing of Mycobacterium tuberculosis: a neglected problem at the turn of the century. Int J Tuberc Lung Dis 1999; 3: 564–581. - PubMed
-
- Dinnes J, Deeks J, Kunst H, et al. . A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess 2007; 11: 1–196. - PubMed
-
- Small PM, Pai M. Tuberculosis diagnosis: time for a game change. N Engl J Med 2010; 363: 1070–1071. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
