Heat stress induces ferroptosis-like cell death in plants
- PMID: 28100685
- PMCID: PMC5294777
- DOI: 10.1083/jcb.201605110
Heat stress induces ferroptosis-like cell death in plants
Abstract
In plants, regulated cell death (RCD) plays critical roles during development and is essential for plant-specific responses to abiotic and biotic stresses. Ferroptosis is an iron-dependent, oxidative, nonapoptotic form of cell death recently described in animal cells. In animal cells, this process can be triggered by depletion of glutathione (GSH) and accumulation of lipid reactive oxygen species (ROS). We investigated whether a similar process could be relevant to cell death in plants. Remarkably, heat shock (HS)-induced RCD, but not reproductive or vascular development, was found to involve a ferroptosis-like cell death process. In root cells, HS triggered an iron-dependent cell death pathway that was characterized by depletion of GSH and ascorbic acid and accumulation of cytosolic and lipid ROS. These results suggest a physiological role for this lethal pathway in response to heat stress in Arabidopsis thaliana The similarity of ferroptosis in animal cells and ferroptosis-like death in plants suggests that oxidative, iron-dependent cell death programs may be evolutionarily ancient.
© 2017 Distéfano et al.
Figures
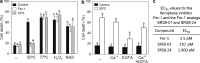
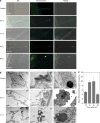




Comment in
-
Back to the roots of regulated necrosis.J Cell Biol. 2017 Feb;216(2):303-304. doi: 10.1083/jcb.201612078. Epub 2017 Jan 20. J Cell Biol. 2017. PMID: 28108525 Free PMC article.
-
Ferroptosis-like cell death in plants.Sci Signal. 2017 Feb 28;10(468):eaan0450. doi: 10.1126/scisignal.aan0450. Sci Signal. 2017. PMID: 28246195
-
Ferroptosis: Yet Another Way to Die.Trends Plant Sci. 2019 Jun;24(6):479-481. doi: 10.1016/j.tplants.2019.03.005. Epub 2019 Mar 23. Trends Plant Sci. 2019. PMID: 30910286
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

