miR-142-3p Is a Key Regulator of IL-1β-Dependent Synaptopathy in Neuroinflammation
- PMID: 28100738
- PMCID: PMC6596761
- DOI: 10.1523/JNEUROSCI.0851-16.2016
miR-142-3p Is a Key Regulator of IL-1β-Dependent Synaptopathy in Neuroinflammation
Abstract
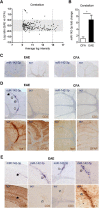
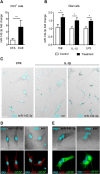
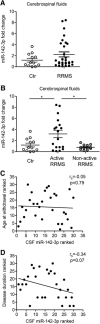
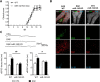
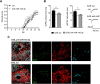
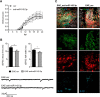
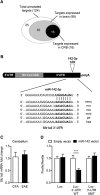
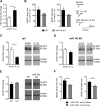
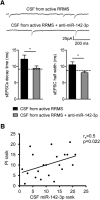
MicroRNAs (miRNA) play an important role in post-transcriptional gene regulation of several physiological and pathological processes. In multiple sclerosis (MS), a chronic inflammatory and degenerative disease of the CNS, and in its mouse model, the experimental autoimmune encephalomyelitis (EAE), miRNA dysregulation has been mainly related to immune system dysfunction and white matter (WM) pathology. However, little is known about their role in gray matter pathology. Here, we explored miRNA involvement in the inflammation-driven alterations of synaptic structure and function, collectively known as synaptopathy, a neuropathological process contributing to excitotoxic neurodegeneration in MS/EAE. Particularly, we observed that miR-142-3p is increased in the CSF of patients with active MS and in EAE brains. We propose miR-142-3p as a molecular mediator of the IL-1β-dependent downregulation of the glial glutamate-aspartate transporter (GLAST), which causes an enhancement of the glutamatergic transmission in the EAE cerebellum. The synaptic abnormalities mediated by IL-1β and the clinical and neuropathological manifestations of EAE disappeared in miR-142 knock-out mice. Furthermore, we observed that in vivo miR-142-3p inhibition, either by a preventive and local treatment or by a therapeutic and systemic strategy, abolished IL-1β- and GLAST-dependent synaptopathy in EAE wild-type mice. Consistently, miR-142-3p was responsible for the glutamatergic synaptic alterations caused by CSF of patients with MS, and CSF levels of miR-142-3p correlated with prospective MS disease progression. Our findings highlight miR-142-3p as key molecular player in IL-1β-mediated synaptic dysfunction, possibly leading to excitotoxic damage in both EAE and MS diseases. Inhibition of miR-142-3p could be neuroprotective in MS.
Significance statement: Current studies suggest the role of glutamate excitotoxicity in the development and progression of multiple sclerosis (MS) and of its mouse model experimental autoimmune encephalomyelitis (EAE). The molecular mechanisms linking inflammation and synaptic alterations in MS/EAE are still unknown. Here, we identified miR-142-3p as a determinant molecular actor in inflammation-dependent synaptopathy typical of both MS and EAE. miR-142-3p was upregulated in the CSF of MS patients and in EAE cerebellum. Inhibition of miR-142-3p, locally in EAE brain and in a MS chimeric ex vivo model, recovered glutamatergic synaptic enhancement typical of EAE/MS. We proved that miR-142-3p promoted the IL-1β-dependent glutamate dysfunction by targeting glutamate-aspartate transporter (GLAST), a crucial glial transporter involved in glutamate homeostasis. Finally, we suggest miR-142-3p as a negative prognostic factor in patients with relapsing-remitting multiple sclerosis.
Keywords: CSF; experimental autoimmune encephalomyelitis; glial glutamate transporter; glutamate excitotoxicity; microRNA; multiple sclerosis.
Copyright © 2017 the authors 0270-6474/17/370547-16$15.00/0.
Figures
References
-
- Ahlbrecht J, Martino F, Pul R, Skripuletz T, Sühs KW, Schauerte C, Yildiz Ö, Trebst C, Tasto L, Thum S, Pfanne A, Roesler R, Lauda F, Hecker M, Zettl UK, Tumani H, Thum T, Stangel M. Deregulation of microRNA-181c in cerebrospinal fluid of patients with clinically isolated syndrome is associated with early conversion to relapsing-remitting multiple sclerosis. Mult Scler. 2016;22:1202–1214. doi: 10.1177/1352458515613641. - DOI - PubMed
-
- Antonucci F, Turola E, Riganti L, Caleo M, Gabrielli M, Perrotta C, Novellino L, Clementi E, Giussani P, Viani P, Matteoli M, Verderio C. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31:1231–1240. doi: 10.1038/emboj.2011.489. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
