Three Small-Receptive-Field Ganglion Cells in the Mouse Retina Are Distinctly Tuned to Size, Speed, and Object Motion
- PMID: 28100743
- PMCID: PMC5242408
- DOI: 10.1523/JNEUROSCI.2804-16.2016
Three Small-Receptive-Field Ganglion Cells in the Mouse Retina Are Distinctly Tuned to Size, Speed, and Object Motion
Abstract
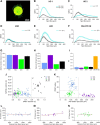
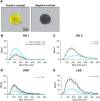
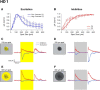
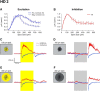
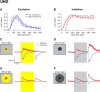
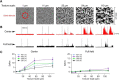
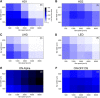
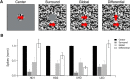
Retinal ganglion cells (RGCs) are frequently divided into functional types by their ability to extract and relay specific features from a visual scene, such as the capacity to discern local or global motion, direction of motion, stimulus orientation, contrast or uniformity, or the presence of large or small objects. Here we introduce three previously uncharacterized, nondirection-selective ON-OFF RGC types that represent a distinct set of feature detectors in the mouse retina. The three high-definition (HD) RGCs possess small receptive-field centers and strong surround suppression. They respond selectively to objects of specific sizes, speeds, and types of motion. We present comprehensive morphological characterization of the HD RGCs and physiological recordings of their light responses, receptive-field size and structure, and synaptic mechanisms of surround suppression. We also explore the similarities and differences between the HD RGCs and a well characterized RGC with a comparably small receptive field, the local edge detector, in response to moving objects and textures. We model populations of each RGC type to study how they differ in their performance tracking a moving object. These results, besides introducing three new RGC types that together constitute a substantial fraction of mouse RGCs, provide insights into the role of different circuits in shaping RGC receptive fields and establish a foundation for continued study of the mechanisms of surround suppression and the neural basis of motion detection.
Significance statement: The output cells of the retina, retinal ganglion cells (RGCs), are a diverse group of ∼40 distinct neuron types that are often assigned "feature detection" profiles based on the specific aspects of the visual scene to which they respond. Here we describe, for the first time, morphological and physiological characterization of three new RGC types in the mouse retina, substantially augmenting our understanding of feature selectivity. Experiments and modeling show that while these three "high-definition" RGCs share certain receptive-field properties, they also have distinct tuning to the size, speed, and type of motion on the retina, enabling them to occupy different niches in stimulus space.
Keywords: feature selectivity; object motion; retina; retinal ganglion cell.
Copyright © 2017 the authors 0270-6474/17/370610-16$15.00/0.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
