Selective Deletion of Sodium Salt Taste during Development Leads to Expanded Terminal Fields of Gustatory Nerves in the Adult Mouse Nucleus of the Solitary Tract
- PMID: 28100747
- PMCID: PMC5242412
- DOI: 10.1523/JNEUROSCI.2913-16.2016
Selective Deletion of Sodium Salt Taste during Development Leads to Expanded Terminal Fields of Gustatory Nerves in the Adult Mouse Nucleus of the Solitary Tract
Abstract
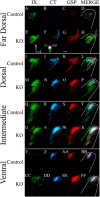
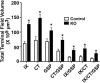
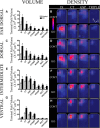
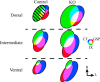
Neuronal activity plays a key role in the development of sensory circuits in the mammalian brain. In the gustatory system, experimental manipulations now exist, through genetic manipulations of specific taste transduction processes, to examine how specific taste qualities (i.e., basic tastes) impact the functional and structural development of gustatory circuits. Here, we used a mouse knock-out model in which the transduction component used to discriminate sodium salts from other taste stimuli was deleted in taste bud cells throughout development. We used this model to test the hypothesis that the lack of activity elicited by sodium salt taste impacts the terminal field organization of nerves that carry taste information from taste buds to the nucleus of the solitary tract (NST) in the medulla. The glossopharyngeal, chorda tympani, and greater superficial petrosal nerves were labeled to examine their terminal fields in adult control mice and in adult mice in which the α-subunit of the epithelial sodium channel was conditionally deleted in taste buds (αENaC knockout). The terminal fields of all three nerves in the NST were up to 2.7 times greater in αENaC knock-out mice compared with the respective field volumes in control mice. The shapes of the fields were similar between the two groups; however, the density and spread of labels were greater in αENaC knock-out mice. Overall, our results show that disruption of the afferent taste signal to sodium salts disrupts the normal age-dependent "pruning" of all terminal fields, which could lead to alterations in sensory coding and taste-related behaviors.
Significance statement: Neural activity plays a major role in the development of sensory circuits in the mammalian brain. To date, there has been no direct test of whether taste-elicited neural activity has a role in shaping central gustatory circuits. However, recently developed genetic tools now allow an assessment of how specific taste stimuli, in this case sodium salt taste, play a role in the maturation of the terminal fields in the mouse brainstem. We found that the specific deletion of sodium salt taste during development produced terminal fields in adults that were dramatically larger than in control mice, demonstrating for the first time that sodium salt taste-elicited activity is necessary for the normal maturation of gustatory inputs into the brain.
Keywords: ENaC; activity; axons; epithelial sodium channel; knock out; medulla.
Copyright © 2017 the authors 0270-6474/17/370660-13$15.00/0.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
