Inverse Relationship between Basal Pacemaker Neuron Activity and Aversive Long-Term Memory Formation in Lymnaea stagnalis
- PMID: 28101006
- PMCID: PMC5209385
- DOI: 10.3389/fncel.2016.00297
Inverse Relationship between Basal Pacemaker Neuron Activity and Aversive Long-Term Memory Formation in Lymnaea stagnalis
Abstract
Learning and memory formation are essential physiological functions. While quiescent neurons have long been the focus of investigations into the mechanisms of memory formation, there is increasing evidence that spontaneously active neurons also play key roles in this process and possess distinct rules of activity-dependent plasticity. In this study, we used a well-defined aversive learning model of aerial respiration in the mollusk Lymnaea stagnalis (L. stagnalis) to study the role of basal firing activity of the respiratory pacemaker neuron Right Pedal Dorsal 1 (RPeD1) as a determinant of aversive long-term memory (LTM) formation. We investigated the relationship between basal aerial respiration behavior and RPeD1 firing activity, and examined aversive LTM formation and neuronal plasticity in animals exhibiting different basal aerial respiration behavior. We report that animals with higher basal aerial respiration behavior exhibited early responses to operant conditioning and better aversive LTM formation. Early behavioral response to the conditioning procedure was associated with biphasic enhancements in the membrane potential, spontaneous firing activity and gain of firing response, with an early phase spanning the first 2 h after conditioning and a late phase that is observed at 24 h. Taken together, we provide the first evidence suggesting that lower neuronal activity at the time of learning may be correlated with better memory formation in spontaneously active neurons. Our findings provide new insights into the diversity of cellular rules of plasticity underlying memory formation.
Keywords: Lymnaea stagnalis; aversive operant conditioning; basal neuronal activity; individual variations in memory formation; neuronal plasticity; spontaneously active neuron.
Figures

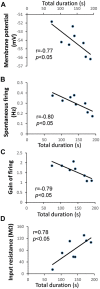
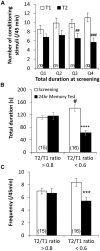
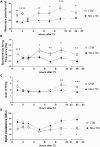

 ) and KCa channel activity (
) and KCa channel activity ( ) (potential modifications signified by yellow circle), and potentiation of firing activity (Nelson et al., 2003, 2005) (C). In contrast, the conditioning stimulus does not significantly induce changes in the spike firing and intracellular Ca2+ concentration in a RPeD1 with higher basal firing activity, such that cellular changes underlying aversive LTM formation do not occur (C). (C) In animals exhibiting low basal RPeD1 firing, the training-induced enhancement in RPeD1 rhythmic firing activity observed at 1 h after training could induce increases in intracellular Ca2+ concentration and consequently activity-dependent signaling pathways (
) (potential modifications signified by yellow circle), and potentiation of firing activity (Nelson et al., 2003, 2005) (C). In contrast, the conditioning stimulus does not significantly induce changes in the spike firing and intracellular Ca2+ concentration in a RPeD1 with higher basal firing activity, such that cellular changes underlying aversive LTM formation do not occur (C). (C) In animals exhibiting low basal RPeD1 firing, the training-induced enhancement in RPeD1 rhythmic firing activity observed at 1 h after training could induce increases in intracellular Ca2+ concentration and consequently activity-dependent signaling pathways ( ), such as CREB (Guo et al., 2010), that trigger aversive LTM formation. At 24 h after training, de novo synthesis of plasticity products (
), such as CREB (Guo et al., 2010), that trigger aversive LTM formation. At 24 h after training, de novo synthesis of plasticity products ( ), such as voltage-gated Ca2+ and non-selective Na+ leak channel (NALCN) leak channels, may serve to maintain enhanced RPeD1 firing activity and aversive LTM.
), such as voltage-gated Ca2+ and non-selective Na+ leak channel (NALCN) leak channels, may serve to maintain enhanced RPeD1 firing activity and aversive LTM.References
-
- Aizenman C. D., Linden D. J. (1999). Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J. Neurophysiol. 82, 1697–1709. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

