Elastase-Induced Parenchymal Disruption and Airway Hyper Responsiveness in Mouse Precision Cut Lung Slices: Toward an Ex vivo COPD Model
- PMID: 28101062
- PMCID: PMC5209351
- DOI: 10.3389/fphys.2016.00657
Elastase-Induced Parenchymal Disruption and Airway Hyper Responsiveness in Mouse Precision Cut Lung Slices: Toward an Ex vivo COPD Model
Abstract
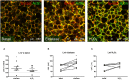
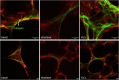
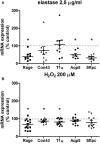
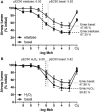
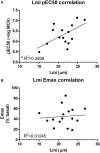
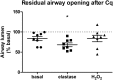
Background: COPD is a progressive lung disease characterized by emphysema and enhanced bronchoconstriction. Current treatments focused on bronchodilation can delay disease progression to some extent, but recovery or normalization of loss of lung function is impossible. Therefore, novel therapeutic targets are needed. The importance of the parenchyma in airway narrowing is increasingly recognized. In COPD, the parenchyma and extracellular matrix are altered, possibly affecting airway mechanics and enhancing bronchoconstriction. Our aim was to set up a comprehensive ex vivo Precision Cut Lung Slice (PCLS) model with a pathophysiology resembling that of COPD and integrate multiple readouts in order to study the relationship between parenchyma, airway functionality, and lung repair processes. Methods: Lungs of C57Bl/6J mice were sliced and treated ex vivo with elastase (2.5 μg/ml) or H2O2 (200 μM) for 16 h. Following treatment, parenchymal structure, airway narrowing, and gene expression levels of alveolar Type I and II cell repair were assessed. Results: Following elastase, but not H2O2 treatment, slices showed a significant increase in mean linear intercept (Lmi), reflective of emphysema. Only elastase-treated slices showed disorganization of elastin and collagen fibers. In addition, elastase treatment lowered both alveolar Type I and II marker expression, whereas H2O2 stimulation lowered alveolar Type I marker expression only. Furthermore, elastase-treated slices showed enhanced methacholine-induced airway narrowing as reflected by increased pEC50 (5.87 at basal vs. 6.50 after elastase treatment) and Emax values (47.96 vs. 67.30%), and impaired chloroquine-induced airway opening. The increase in pEC50 correlated with an increase in mean Lmi. Conclusion: Using this model, we show that structural disruption of elastin fibers leads to impaired alveolar repair, disruption of the parenchymal compartment, and altered airway biomechanics, enhancing airway contraction. This finding may have implications for COPD, as the amount of elastin fiber and parenchymal tissue disruption is associated with disease severity. Therefore, we suggest that PCLS can be used to model certain aspects of COPD pathophysiology and that the parenchymal tissue damage observed in COPD contributes to lung function decline by disrupting airway biomechanics. Targeting the parenchymal compartment may therefore be a promising therapeutic target in the treatment of COPD.
Keywords: airway mechanics; chronic obstructive pulmonary disease; extracellular matrix.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

