A Disease Model of Muscle Necrosis Caused by Aeromonas dhakensis Infection in Caenorhabditis elegans
- PMID: 28101079
- PMCID: PMC5209350
- DOI: 10.3389/fmicb.2016.02058
A Disease Model of Muscle Necrosis Caused by Aeromonas dhakensis Infection in Caenorhabditis elegans
Abstract
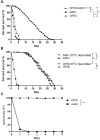
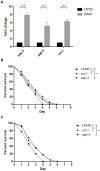
A variety of bacterial infections cause muscle necrosis in humans. Caenorhabditis elegans has epidermis and bands of muscle that resemble soft-tissue structures in mammals and humans. Here, we developed a muscle necrosis model caused by Aeromonas dhakensis infection in C. elegans. Our data showed that A. dhakensis infected and killed C. elegans rapidly. Characteristic muscle damage in C. elegans induced by A. dhakensis was demonstrated in vivo. Relative expression levels of host necrosis-associated genes, asp-3, asp-4, and crt-1 increased significantly after A. dhakensis infection. The RNAi sensitive NL2099 rrf-3 (pk1426) worms with knockdown of necrosis genes of crt-1 and asp-4 by RNAi showed prolonged survival after A. dhakensis infection. Specifically knockdown of crt-1 and asp-4 by RNAi in WM118 worms, which restricted RNAi only to the muscle cells, conferred significant resistance to A. dhakensis infection. In contrast, the severity of muscle damage and toxicity produced by the A. dhakensis hemolysin-deletion mutant is attenuated. In another example, shiga-like toxin-producing enterohemorrhagic E. coli (EHEC) known to elicit toxicity to C. elegans with concomitant enteropathogenicty, did not cause muscle necrosis as A. dhakensis did. Taken together, these results show that Aeromonas infection induces muscle necrosis and rapid death of infected C. elegans, which are similar to muscle necrosis in humans, and then validate the value of the C. elegans model with A. dhakensis infection in studying Aeromonas pathogenicity.
Keywords: Aeromonas dhakensis; Caenorhabditis elegans; disease model; infection; muscle necrosis.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

