Bone health in HIV and hepatitis B or C infections
- PMID: 28101146
- PMCID: PMC5228639
- DOI: 10.1177/1759720X16671927
Bone health in HIV and hepatitis B or C infections
Abstract
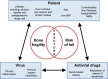
Chronic infections with human immunodeficiency virus (HIV), hepatitis B virus (HBV) or hepatitis C virus (HCV) add to age-dependent bone loss and may contribute to lower bone strength in the elderly. In this review, we report recent highlights on the epidemiology of bone fragility in chronic viral infections with HIV, HCV and HBV, its physiopathology and discuss the interference of antiviral therapies with bone metabolism. Chronic infections influence bone through the interactions between risk factors for bone fragility and falls (which are highly prevalent in infected patients), virus activity and antiviral drugs. HIV-infected patients are at increased risk of fracture and the risk is higher in cases of co-infection with HIV and untreated chronic viral hepatitis. In HIV patients, the majority of bone loss occurs during virus activity and at initiation of antiretroviral therapy (ART). However, long-term elderly HIV-infected patients on successful ART display bone microstructure alterations only partially captured by dual energy X-ray absorptiometry (DXA). Bone loss is associated with an increase of bone resorption, reflecting the upregulation of the receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoprotegerin (OPG) pathways via a crosstalk between virus activity, inflammation and the immune system. The use of some antiviral drugs, such as tenofovir (controlling both HBV and HIV infections) or protease inhibitors, may be associated with higher bone toxicity. The reduction of tenofovir plasma concentrations with the implementation of tenofovir alafenamide (TAF) attenuates bone mineral density (BMD) loss but it remains unknown whether it will contribute to reducing fracture risk in long-term HIV-treated patients. Moreover, to what extent the new direct-acting agents for treatment of HCV, including nucleotide inhibitors and protease inhibitors, may affect bone health similarly as ART in HIV should be investigated.
Keywords: HIV; bone mineral density; fracture; hepatitis B; hepatitis C; osteoporosis.
Conflict of interest statement
The authors declare that there is no conflict of interest. AC has received unrestricted educational and research grant from GILEAD Switzerland, and unrestricted educational grants from AbbVie, ViiV Healthcare, MSD, BMS, and Janssen-Cilag Switzerland (HIV Unit).
Figures
References
-
- Baeg M., Yoon S., Ko S., Han K., Choi H., Bae S., et al. (2016) Males seropositive for hepatitis B surface antigen are at risk of lower bone mineral density: the 2008–2010 Korea National Health and Nutrition Examination Surveys. Hepatol Int 10: 470–477. - PubMed
-
- Bernardino J., Mocroft A., Mallon P., Wallet C., Gerstoft J., Russell C., et al. (2015) Bone mineral density and inflammatory and bone biomarkers after darunavir-ritonavir combined with either raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults with HIV-1: a substudy of the NEAT001/ANRS143 randomised trial. Lancet HIV 2: e464–e473. - PubMed
-
- Biver E., Calmy A., Delhumeau C., Durosier C., Zawadynski S., Rizzoli R. (2014) Microstructural alterations of trabecular and cortical bone in long-term HIV-infected elderly men on successful antiretroviral therapy. AIDS 28: 2417–2427. - PubMed
-
- Bolland M., Grey A., Reid I. (2015) Skeletal health in adults with HIV infection. Lancet Diabetes Endocrinol 3: 63–74. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

