Antifungal and anthelmintic activity of novel benzofuran derivatives containing thiazolo benzimidazole nucleus: an in vitro evaluation
- PMID: 28101251
- PMCID: PMC5218922
- DOI: 10.1007/s12154-016-0160-x
Antifungal and anthelmintic activity of novel benzofuran derivatives containing thiazolo benzimidazole nucleus: an in vitro evaluation
Abstract
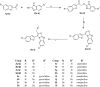
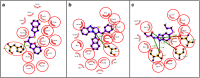
A novel series of thiazolo[3,2-a]benzimidazole derivatives containing benzofuran nucleus (5a-l) have been synthesized. The key intermediate, substituted benzimidazol-sulfanyl benzofuran ethanone (3a-d) was prepared by refluxing the mixture of substituted 2-acetyl benzofuran and substituted 2-mercaptobenzimidazole in acetic acid. The cyclisation of compounds (3a-d) using polyphosphoric acid furnished the corresponding 6-substituted benzofuran thiazolo[3,2-a]benzimidazoles (4a-d). Further, the cyclized compounds (4a-d) were subjected for Mannich reaction to give corresponding Mannich bases (5a-l). All newly synthesized compounds were screened for antifungal and anthelmintic activity. Amongst the tested compounds, 4b and 4d exhibited potential antifungal activity. From the anthelmintic activity data, it was found that the compounds 3a, 3b and 5i were found to be more effective against the tested earthworm Pheretima posthuma. In correlation to anthelmintic activity, the selected compounds were subjected for molecular docking studies and the compounds 3a and 5i have emerged as active anthelmintic agents with maximum binding affinity (-3.7 and -5.4 kcal/mol).
Keywords: Anthelmintic activity; Antifungal; Benzimidazole; Benzofuran; Mannich base; Molecular docking; β-Tubulin.
Figures

Similar articles
-
Synthesis, Characterization and Molecular Docking Studies of Novel N-(benzimidazol-1-ylmethyl)-4-chlorobenzamide Analogues for Potential Anti-inflammatory and Antimicrobial Activity.Antiinflamm Antiallergy Agents Med Chem. 2018;17(1):16-31. doi: 10.2174/1871523017666180426125141. Antiinflamm Antiallergy Agents Med Chem. 2018. PMID: 29697033
-
Synthesis of novel benzofuran and related benzimidazole derivatives for evaluation of in vitro anti-HIV-1, anticancer and antimicrobial activities.Arch Pharm Res. 2006 Oct;29(10):826-33. doi: 10.1007/BF02973901. Arch Pharm Res. 2006. PMID: 17121175
-
Synthesis of Some Benzofuran Derivatives Containing Pyrimidine Moiety as Potent Antimicrobial Agents.Iran J Pharm Res. 2018 Winter;17(1):75-86. Iran J Pharm Res. 2018. PMID: 29755540 Free PMC article.
-
Mannich bases: an important pharmacophore in present scenario.Int J Med Chem. 2014;2014:191072. doi: 10.1155/2014/191072. Epub 2014 Nov 12. Int J Med Chem. 2014. PMID: 25478226 Free PMC article. Review.
-
Design and synthesis of some substituted thiazolo[3,2-a]pyrimidine derivatives of potential biological activities.Saudi Pharm J. 2016 Mar;24(2):119-32. doi: 10.1016/j.jsps.2013.12.016. Epub 2013 Dec 28. Saudi Pharm J. 2016. PMID: 27013904 Free PMC article. Review.
Cited by
-
Synthesis and Antimicrobial Activity of New Heteroaryl(aryl) Thiazole Derivatives Molecular Docking Studies.Antibiotics (Basel). 2022 Sep 30;11(10):1337. doi: 10.3390/antibiotics11101337. Antibiotics (Basel). 2022. PMID: 36289995 Free PMC article.
-
Synthesis of Surfactants Derived from 2-Mercaptobenzimidazole and Study of Their Acute Toxicity and Analgesic and Psychotropic Activities.Biochem Res Int. 2019 Jul 30;2019:9615728. doi: 10.1155/2019/9615728. eCollection 2019. Biochem Res Int. 2019. PMID: 31467714 Free PMC article.
-
5-Benzyliden-2-(5-methylthiazol-2-ylimino)thiazolidin-4-ones as Antimicrobial Agents. Design, Synthesis, Biological Evaluation and Molecular Docking Studies.Antibiotics (Basel). 2021 Mar 17;10(3):309. doi: 10.3390/antibiotics10030309. Antibiotics (Basel). 2021. PMID: 33802949 Free PMC article.
-
Benzimidazole(s): synthons, bioactive lead structures, total synthesis, and the profiling of major bioactive categories.RSC Adv. 2025 Mar 28;15(10):7571-7608. doi: 10.1039/d4ra08864f. eCollection 2025 Mar 6. RSC Adv. 2025. PMID: 40161353 Free PMC article. Review.
-
Molecular docking and dynamic simulations of benzimidazoles with beta-tubulins.Bioinformation. 2021 Mar 31;17(3):404-412. doi: 10.6026/97320630017404. eCollection 2021. Bioinformation. 2021. PMID: 34092961 Free PMC article.
References
-
- Ravindernath A, Reddy MS (2013) Synthesis and evaluation of anti-inflammatory, antioxidant and antimicrobial activities of densely functionalized novel benzo [d] imidazolyl Tetrahydropyridine carboxylates. doi:10.1016/j.arabjc.2013.02.011
-
- Premakumari C, Muralikrishna A, Padmaja A, Padmavathi V, Park SJ, Kim TJ, Reddy GD. Synthesis, antimicrobial and anticancer activities of amido sulfonamido methane linked bis heterocycles. Ara J Chem. 2014;7:385–395. doi: 10.1016/j.arabjc.2013.10.024. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

