Variation in dose and plasma level of lamotrigine in patients discharged from a mental health trust
- PMID: 28101320
- PMCID: PMC5228716
- DOI: 10.1177/2045125316672573
Variation in dose and plasma level of lamotrigine in patients discharged from a mental health trust
Abstract
Background: The objectives of this study were to investigate the dose of lamotrigine when prescribed with an enzyme inhibitor or enzyme inducer in patients discharged from a mental health trust and to determine the corresponding lamotrigine plasma concentrations and the factors that may affect these.
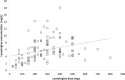
Methods: All patients discharged on lamotrigine between October 2007 and September 2012 were identified using the pharmacy dispensing database. We recorded demographic details, lamotrigine dose and plasma levels and coprescribed medication.
Results: During the designated period, 187 patients were discharged on lamotrigine of whom 117 had their plasma levels recorded. The mean lamotrigine daily dose was 226.1 mg (range 12.5-800 mg) and the mean plasma level 5.9 mg/l (range 0.8-18.1 mg/l). Gender, ethnicity, diagnosis and smoking status had no significant effect on dose or plasma levels. Patients taking an enzyme-inducing drug (n = 6) had significantly lower plasma levels [mean (SD) 3.40 (1.54) mg/l] than those not taking enzyme inducers [n = 111; 6.03 (3.13) mg/l; p = 0.043]. Patients taking an enzyme-inhibiting drug (n = 23) had significantly higher levels [7.47 (3.99) mg/l] than those not taking an inhibitor [n = 94; 5.52 (2.75) mg/l; p = 0.035]. No significant difference was found between the doses of lamotrigine in patients taking an enzyme inhibitor and those not taking one (p = 0.376). No significant difference was found between the doses of lamotrigine in patients taking an enzyme-inducing drug and those not taking any (p = 0.574).
Conclusions: Current dosing recommendations indicate that lamotrigine doses should be halved in individuals taking enzyme inhibitors and doubled in those on enzyme inducers. In our survey these recommendations were rarely followed with the consequence that patients received too high or too low a dose of lamotrigine, respectively.
Keywords: drug interactions; lamotrigine; therapeutic drug monitoring.
Conflict of interest statement
DT has received payments for lectures and advisory boards from Eli Lilly, Lundbeck, Bristol Myers Squibb, Astra Zeneca, Sunovion and Otsuka. FG has received payments for lectures and advisory boards from Lundbeck, Roche, Sunovion, Bristol Myers Squibb and Otsuka and has a family member with professional links to Eli Lilly and GlaxoSmithKline. Other authors declare no potential conflicts of interest.
Figures
References
-
- Anderson G., Yau M., Gidal B., Harris S., Levy R., Lai A., et al. (1996) Bidirectional interaction of valproate and lamotrigine in healthy subjects. Clin Pharmacol Ther 60: 145–156. - PubMed
-
- Cohen A., Land G., Breimer D., Yuen W., Winton C., Peck A. (1987) Lamotrigine, a new anticonvulsant: pharmacokinetics in normal humans. Clin Pharmacol Ther 42: 535–541. - PubMed
-
- GlaxoSmithKline UK. (2016) Summary of Product Characteristics: Lamictal. Available at: https://www.medicines.org.uk/emc/medicine/4228
-
- Hiemke C., Baumann P., Bergemann N., Conca A., Dietmaier O., Egberts K., et al. (2011) AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry 44: 195–235. - PubMed
-
- Johannessen S., Battino D., Berry D., Bialer M., Kramer G., Tomson T., et al. (2003) Therapeutic drug monitoring of the newer antiepileptic drugs. Ther Drug Monit 25: 347–363. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources