Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration
- PMID: 28101337
- PMCID: PMC5237166
- DOI: 10.1186/s40170-016-0163-7
Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration
Abstract
Background: Obesity is associated with increased recurrence and reduced survival of breast cancer. Adipocytes constitute a significant component of breast tissue, yet their role in provisioning metabolic substrates to support breast cancer progression is poorly understood.
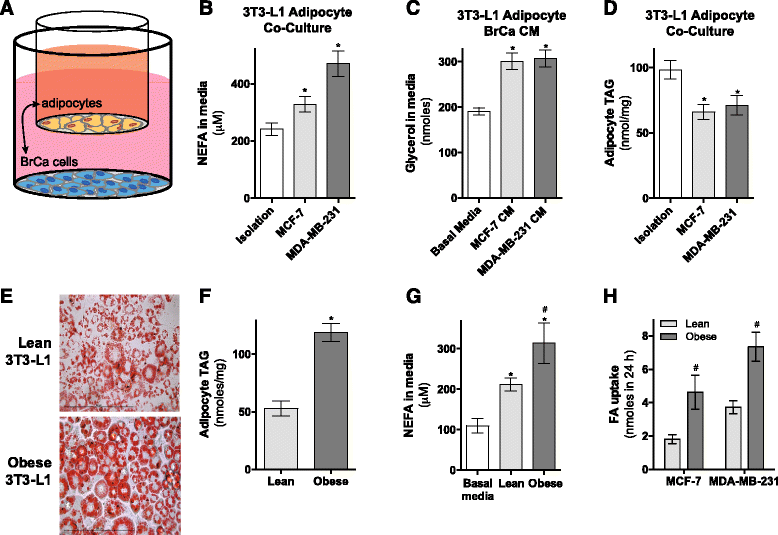
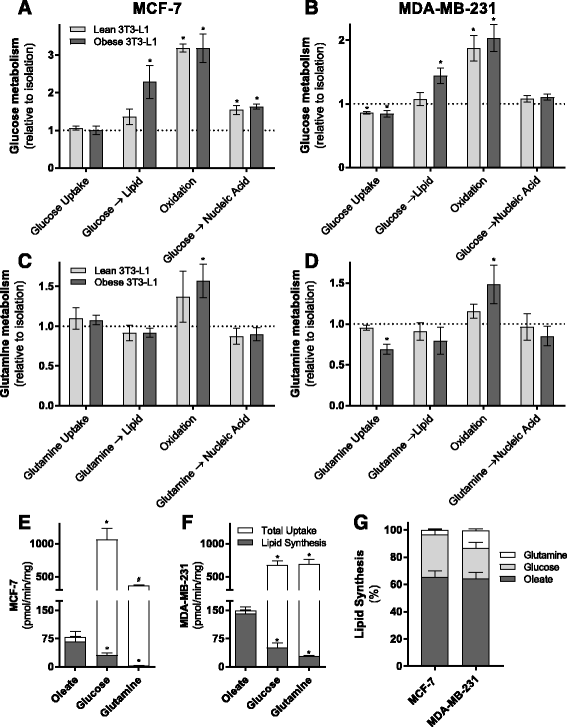
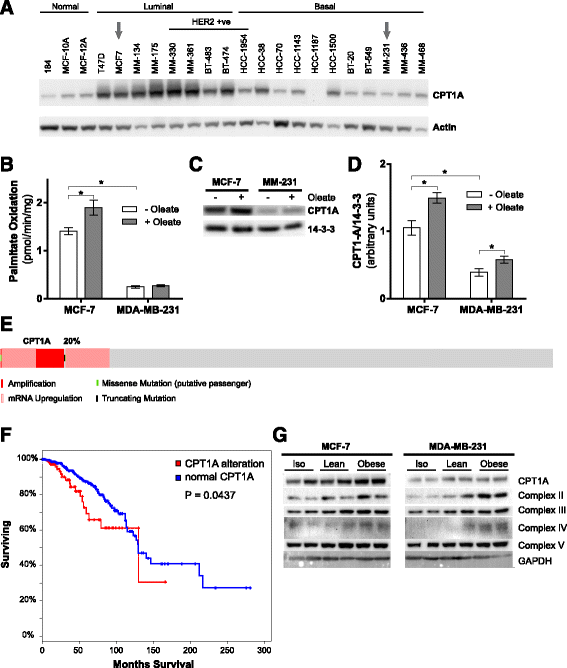
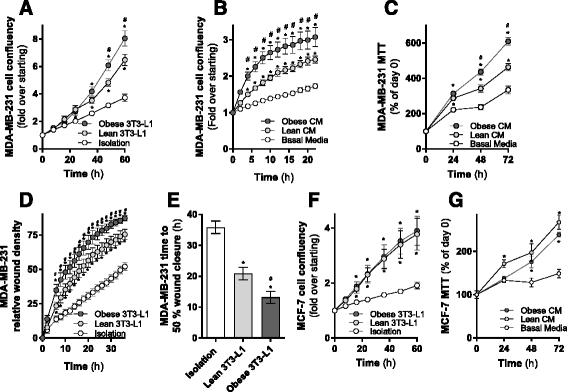
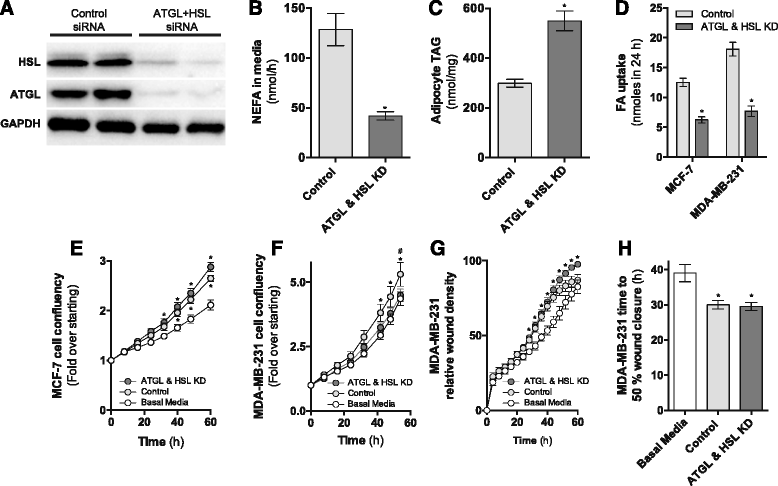
Results: Here, we show that co-culture of breast cancer cells with adipocytes revealed cancer cell-stimulated depletion of adipocyte triacylglycerol. Adipocyte-derived free fatty acids were transferred to breast cancer cells, driving fatty acid metabolism via increased CPT1A and electron transport chain complex protein levels, resulting in increased proliferation and migration. Notably, fatty acid transfer to breast cancer cells was enhanced from "obese" adipocytes, concomitant with increased stimulation of cancer cell proliferation and migration. This adipocyte-stimulated breast cancer cell proliferation was dependent on lipolytic processes since HSL/ATGL knockdown attenuated cancer cell responses.
Conclusions: These findings highlight a novel and potentially important role for adipocyte lipolysis in the provision of metabolic substrates to breast cancer cells, thereby supporting cancer progression.
Keywords: Adipocytes; Breast cancer; Lipid metabolism; Metabolic crosstalk; Obesity.
Figures

References
-
- Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14(1):11-31. doi:10.1038/nrclinonc.2016.60. [PMID: 27141887]. - PubMed
-
- Yoon S, Lee MY, Park SW, Moon JS, Koh YK, Ahn YH, Park BW, Kim KS. Up-regulation of Acetyl-CoA carboxylase alpha and fatty acid synthase by human epidermal growth factor receptor 2 at the translational level in breast cancer cells. J Biol Chem. 2007;282:26122–31. doi: 10.1074/jbc.M702854200. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

