Structural and functional human retinal imaging with a fiber-based visible light OCT ophthalmoscope
- PMID: 28101421
- PMCID: PMC5231302
- DOI: 10.1364/BOE.8.000323
Structural and functional human retinal imaging with a fiber-based visible light OCT ophthalmoscope
Abstract
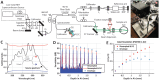
The design of a multi-functional fiber-based Optical Coherence Tomography (OCT) system for human retinal imaging with < 2 micron axial resolution in tissue is described. A detailed noise characterization of two supercontinuum light sources with different pulse repetition rates is presented. The higher repetition rate and lower noise source is found to enable a sensitivity of 96 dB with 0.15 mW light power at the cornea and a 98 microsecond exposure time. Using a broadband (560 ± 50 nm), 90/10, fused single-mode fiber coupler designed for visible wavelengths, the sample arm is integrated into an ophthalmoscope platform, similar to current clinical OCT systems. To demonstrate the instrument's range of operation, in vivo structural retinal imaging is also shown at 0.15 mW exposure with 10,000 and 70,000 axial scans per second (the latter comparable to commercial OCT systems), and at 0.03 mW exposure and 10,000 axial scans per second (below maximum permissible continuous exposure levels). Lastly, in vivo spectroscopic imaging of anatomy, saturation, and hemoglobin content in the human retina is also demonstrated.
Keywords: (060.2350) Fiber optics imaging; (140.7300) Visible lasers; (170.3880) Medical and biological imaging; (170.4500) Optical coherence tomography; (170.6480) Spectroscopy, speckle.
Figures






References
-
- Schuman J. S., Puliafito C. A., Fujimoto J. G., Duker J. S., Optical Coherence Tomography of Ocular Diseases, 3rd ed. (SLACK Inc., Thorofare, NJ, 2013), pp. xviii, 615.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
