Non-invasive terahertz imaging of tissue water content for flap viability assessment
- PMID: 28101431
- PMCID: PMC5231313
- DOI: 10.1364/BOE.8.000460
Non-invasive terahertz imaging of tissue water content for flap viability assessment
Abstract
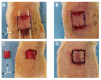
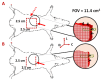
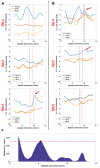
Accurate and early prediction of tissue viability is the most significant determinant of tissue flap survival in reconstructive surgery. Perturbation in tissue water content (TWC) is a generic component of the tissue response to such surgeries, and, therefore, may be an important diagnostic target for assessing the extent of flap viability in vivo. We have previously shown that reflective terahertz (THz) imaging, a non-ionizing technique, can generate spatially resolved maps of TWC in superficial soft tissues, such as cornea and wounds, on the order of minutes. Herein, we report the first in vivo pilot study to investigate the utility of reflective THz TWC imaging for early assessment of skin flap viability. We obtained longitudinal visible and reflective THz imagery comparing 3 bipedicled flaps (i.e. survival model) and 3 fully excised flaps (i.e. failure model) in the dorsal skin of rats over a postoperative period of 7 days. While visual differences between both models manifested 48 hr after surgery, statistically significant (p < 0.05, independent t-test) local differences in TWC contrast were evident in THz flap image sets as early as 24 hr. Excised flaps, histologically confirmed as necrotic, demonstrated a significant, yet localized, reduction in TWC in the flap region compared to non-traumatized skin. In contrast, bipedicled flaps, histologically verified as viable, displayed mostly uniform, unperturbed TWC across the flap tissue. These results indicate the practical potential of THz TWC sensing to accurately predict flap failure 24 hours earlier than clinical examination.
Keywords: (110.0110) Imaging systems; (170.0170) Medical optics and biotechnology; (170.6795) Terahertz imaging.
Figures



References
-
- Payette J. R., Kohlenberg E., Leonardi L., Pabbies A., Kerr P., Liu K.-Z., Sowa M. G., “Assessment of Skin Flaps Using Optically Based Methods for Measuring Blood Flow and Oxygenation,” Plast. Reconstr. Surg. 115(2), 539–546 (2005).10.1097/01.PRS.0000148415.54546.CA - DOI - PubMed
-
- Di Sieno L., Bettega G., Berger M., Hamou C., Aribert M., Mora A. D., Puszka A., Grateau H., Contini D., Hervé L., Coll J.-L., Dinten J.-M., Pifferi A., Planat-Chrétien A., “Toward noninvasive assessment of flap viability with time-resolved diffuse optical tomography: a preclinical test on rats,” J. Biomed. Opt. 21(2), 025004 (2016).10.1117/1.JBO.21.2.025004 - DOI - PubMed
-
- Yang Q., Ren Z. H., Chickooree D., Wu H. J., Tan H. Y., Wang K., He Z. J., Gong C. J., Ram V., Zhang S., “The effect of early detection of anterolateral thigh free flap crisis on the salvage success rate, based on 10 years of experience and 1072 flaps,” Int. J. Oral Maxillofac. Surg. 43(9), 1059–1063 (2014).10.1016/j.ijom.2014.06.003 - DOI - PubMed
-
- Chen K.-T., Mardini S., Chuang D. C.-C., Lin C.-H., Cheng M.-H., Lin Y.-T., Huang W.-C., Tsao C.-K., Wei F.-C., “Timing of presentation of the first signs of vascular compromise dictates the salvage outcome of free flap transfers,” Plast. Reconstr. Surg. 120(1), 187–195 (2007).10.1097/01.prs.0000264077.07779.50 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
