Use of sonic tomography to detect and quantify wood decay in living trees
- PMID: 28101433
- PMCID: PMC5238698
- DOI: 10.3732/apps.1600060
Use of sonic tomography to detect and quantify wood decay in living trees
Abstract
Premise of the study: Field methodology and image analysis protocols using acoustic tomography were developed and evaluated as a tool to estimate the amount of internal decay and damage of living trees, with special attention to tropical rainforest trees with irregular trunk shapes.
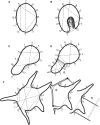
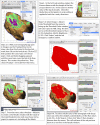
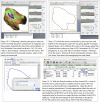
Methods and results: Living trunks of a diversity of tree species in tropical rainforests in the Republic of Panama were scanned using an Argus Electronic PiCUS 3 Sonic Tomograph and evaluated for the amount and patterns of internal decay. A protocol using ImageJ analysis software was used to quantify the proportions of intact and compromised wood. The protocols provide replicable estimates of internal decay and cavities for trees of varying shapes, wood density, and bark thickness.
Conclusions: Sonic tomography, coupled with image analysis, provides an efficient, noninvasive approach to evaluate decay patterns and structural integrity of even irregularly shaped living trees.
Keywords: Argus PiCUS 3 Sonic Tomograph; ImageJ; acoustic tomography; tropical trees.
Figures




References
-
- Bethge K., Mattheck C., Hunger E. 1996. Equipment for detection and evaluation of incipient decay in trees. Arboricultural Journal 20: 13–37.
-
- Costello L., Quarles S. 1999. Detection of wood decay in blue gum and elm: An evaluation of the Resistograph and the portable drill. Journal of Arboriculture 25: 311–317.
-
- Deflorio G., Fink S., Schwarze F. W. M. R. 2008. Detection of incipient decay in tree stems with sonic tomography after wounding and fungal inoculation. Wood Science and Technology 42: 117–132.
-
- Donnelly D. J., Davison E. M. 2008. Comparison of the occurrence of rot in sawlogs from regrowth and mature stands of Eucalyptus diversicolor (karri). Australian Forestry 71: 27–32.
-
- Gilbert E. A., Smiley E. T. 2004. Picus sonic tomography for the quantification of decay in white oak (Quercus alba) and hickory (Carya spp.). Journal of Arboriculture 30: 277–280.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

