Plasma Membrane Targeting of Endogenous NKCC2 in COS7 Cells Bypasses Functional Golgi Cisternae and Complex N-Glycosylation
- PMID: 28101499
- PMCID: PMC5209364
- DOI: 10.3389/fcell.2016.00150
Plasma Membrane Targeting of Endogenous NKCC2 in COS7 Cells Bypasses Functional Golgi Cisternae and Complex N-Glycosylation
Abstract
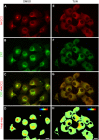
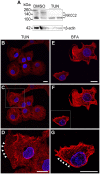
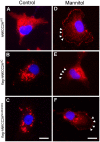
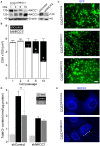
Na+K+2Cl- co-transporters (NKCCs) effect the electroneutral movement of Na+-K+ and 2Cl- ions across the plasma membrane of vertebrate cells. There are two known NKCC isoforms, NKCC1 (Slc12a2) and NKCC2 (Slc12a1). NKCC1 is a ubiquitously expressed transporter involved in cell volume regulation, Cl- homeostasis and epithelial salt secretion, whereas NKCC2 is abundantly expressed in kidney epithelial cells of the thick ascending loop of Henle, where it plays key roles in NaCl reabsorption and electrolyte homeostasis. Although NKCC1 and NKCC2 co-transport the same ions with identical stoichiometry, NKCC1 actively co-transports water whereas NKCC2 does not. There is growing evidence showing that NKCC2 is expressed outside the kidney, but its function in extra-renal tissues remains unknown. The present study shows molecular and functional evidence of endogenous NKCC2 expression in COS7 cells, a widely used mammalian cell model. Endogenous NKCC2 is primarily found in recycling endosomes, Golgi cisternae, Golgi-derived vesicles, and to a lesser extent in the endoplasmic reticulum. Unlike NKCC1, NKCC2 is minimally hybrid/complex N-glycosylated under basal conditions and yet it is trafficked to the plasma membrane region of hyper-osmotically challenged cells through mechanisms that require minimal complex N-glycosylation or functional Golgi cisternae. Control COS7 cells exposed to slightly hyperosmotic (~6.7%) solutions for 16 h were not shrunken, suggesting that either one or both NKCC1 and NKCC2 may participate in cell volume recovery. However, NKCC2 targeted to the plasma membrane region or transient over-expression of NKCC2 failed to rescue NKCC1 in COS7 cells where NKCC1 had been silenced. Further, COS7 cells in which NKCC1, but not NKCC2, was silenced exhibited reduced cell size compared to control cells. Altogether, these results suggest that NKCC2 does not participate in cell volume recovery and therefore, NKCC1 and NKCC2 are functionally different Na+K+2Cl- co-transporters.
Keywords: COS7; Golgi; N-glycosylation; NKCC2A; cell volume.
Figures
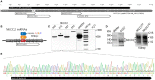
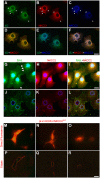
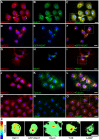
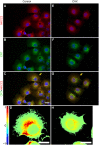
Similar articles
-
Impact of Hybrid and Complex N-Glycans on Cell Surface Targeting of the Endogenous Chloride Cotransporter Slc12a2.Int J Cell Biol. 2015;2015:505294. doi: 10.1155/2015/505294. Epub 2015 Aug 17. Int J Cell Biol. 2015. PMID: 26351455 Free PMC article.
-
Increased Slc12a1 expression in β-cells and improved glucose disposal in Slc12a2 heterozygous mice.J Endocrinol. 2015 Dec;227(3):153-65. doi: 10.1530/JOE-15-0327. Epub 2015 Sep 23. J Endocrinol. 2015. PMID: 26400961 Free PMC article.
-
Comparison of Na-K-Cl cotransporters. NKCC1, NKCC2, and the HEK cell Na-L-Cl cotransporter.J Biol Chem. 1998 May 1;273(18):11295-301. doi: 10.1074/jbc.273.18.11295. J Biol Chem. 1998. PMID: 9556622
-
Physiology and pathophysiology of SLC12A1/2 transporters.Pflugers Arch. 2014 Jan;466(1):91-105. doi: 10.1007/s00424-013-1370-5. Epub 2013 Oct 6. Pflugers Arch. 2014. PMID: 24097229 Free PMC article. Review.
-
The role of Na-K-Cl co-transporter in cerebral ischemia.Neurol Res. 2005 Apr;27(3):280-6. doi: 10.1179/016164105X25243. Neurol Res. 2005. PMID: 15845211 Review.
Cited by
-
Inefficient maturation of disease-linked mutant forms of the KCC2 potassium-chloride cotransporter correlates with predicted pathogenicity.J Biol Chem. 2025 Apr;301(4):108399. doi: 10.1016/j.jbc.2025.108399. Epub 2025 Mar 10. J Biol Chem. 2025. PMID: 40074080 Free PMC article.
-
The neuronal K+Cl- co-transporter 2 (Slc12a5) modulates insulin secretion.Sci Rep. 2017 May 11;7(1):1732. doi: 10.1038/s41598-017-01814-0. Sci Rep. 2017. PMID: 28496181 Free PMC article.
-
New hemisynthetic derivatives of sphaeropsidin phytotoxins triggering severe endoplasmic reticulum swelling in cancer cells.Sci Rep. 2024 Jun 25;14(1):14674. doi: 10.1038/s41598-024-65335-3. Sci Rep. 2024. PMID: 38918539 Free PMC article.
-
The biogenesis of potassium transporters: implications of disease-associated mutations.Crit Rev Biochem Mol Biol. 2024 Jun-Aug;59(3-4):154-198. doi: 10.1080/10409238.2024.2369986. Epub 2024 Jul 1. Crit Rev Biochem Mol Biol. 2024. PMID: 38946646 Free PMC article. Review.
-
The FSGS protein actinin-4 interacts with NKCC2 to regulate thick ascending limb NaCl reabsorption.Am J Physiol Renal Physiol. 2024 Dec 1;327(6):F1026-F1036. doi: 10.1152/ajprenal.00119.2024. Epub 2024 Oct 24. Am J Physiol Renal Physiol. 2024. PMID: 39446130 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

