Rapamycin-Resistant mTOR Activity Is Required for Sensory Axon Regeneration Induced by a Conditioning Lesion
- PMID: 28101526
- PMCID: PMC5234127
- DOI: 10.1523/ENEURO.0358-16.2016
Rapamycin-Resistant mTOR Activity Is Required for Sensory Axon Regeneration Induced by a Conditioning Lesion
Abstract
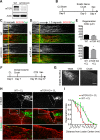
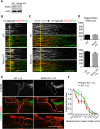
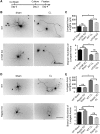
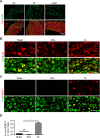
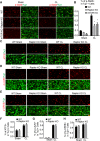
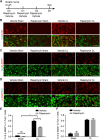
Neuronal mammalian target of rapamycin (mTOR) activity is a critical determinant of the intrinsic regenerative ability of mature neurons in the adult central nervous system (CNS). However, whether its action also applies to peripheral nervous system (PNS) neurons after injury remains elusive. To address this issue unambiguously, we used genetic approaches to determine the role of mTOR signaling in sensory axon regeneration in mice. We showed that deleting mTOR in dorsal root ganglion (DRG) neurons suppressed the axon regeneration induced by conditioning lesions. To establish whether the impact of mTOR on axon regeneration results from functions of mTOR complex 1 (mTORC1) or 2 (mTORC2), two distinct kinase complexes, we ablated either Raptor or Rictor in DRG neurons. We found that suppressing mTORC1 signaling dramatically decreased the conditioning lesion effect. In addition, an injury to the peripheral branch boosts mTOR activity in DRG neurons that cannot be completely inhibited by rapamycin, a widely used mTOR-specific inhibitor. Unexpectedly, examining several conditioning lesion-induced pro-regenerative pathways revealed that Raptor deletion but not rapamycin suppressed Stat3 activity in neurons. Therefore, our results demonstrate that crosstalk between mTOR and Stat3 signaling mediates the conditioning lesion effect and provide genetic evidence that rapamycin-resistant mTOR activity contributes to the intrinsic axon growth capacity in adult sensory neurons after injury.
Keywords: Stat3; axon regeneration; conditioning lesion; dorsal root ganglion; mTOR; spinal cord injury.
Conflict of interest statement
Authors report no conflict of interest.
Figures



Similar articles
-
mTOR signaling pathway differently regulates central and peripheral axon regeneration.Acta Biochim Biophys Sin (Shanghai). 2017 Aug 1;49(8):689-695. doi: 10.1093/abbs/gmx068. Acta Biochim Biophys Sin (Shanghai). 2017. PMID: 28666378
-
Syntaxin13 expression is regulated by mammalian target of rapamycin (mTOR) in injured neurons to promote axon regeneration.J Biol Chem. 2014 May 30;289(22):15820-32. doi: 10.1074/jbc.M113.536607. Epub 2014 Apr 15. J Biol Chem. 2014. PMID: 24737317 Free PMC article.
-
mTORC1 and mTORC2 regulate insulin secretion through Akt in INS-1 cells.J Endocrinol. 2013 Jan 2;216(1):21-9. doi: 10.1530/JOE-12-0351. Print 2013 Jan. J Endocrinol. 2013. PMID: 23092880
-
The Role of Mammalian Target of Rapamycin (mTOR) in Insulin Signaling.Nutrients. 2017 Oct 27;9(11):1176. doi: 10.3390/nu9111176. Nutrients. 2017. PMID: 29077002 Free PMC article. Review.
-
Targeted Inhibition of Rictor/mTORC2 in Cancer Treatment: A New Era after Rapamycin.Curr Cancer Drug Targets. 2016;16(4):288-304. doi: 10.2174/1568009616666151113120830. Curr Cancer Drug Targets. 2016. PMID: 26563881 Review.
Cited by
-
The RSK2-RPS6 axis promotes axonal regeneration in the peripheral and central nervous systems.PLoS Biol. 2023 Apr 17;21(4):e3002044. doi: 10.1371/journal.pbio.3002044. eCollection 2023 Apr. PLoS Biol. 2023. PMID: 37068088 Free PMC article.
-
Ultrasound Stimulation Increases Neurite Regeneration in Injured Dorsal Root Ganglion Neurons through Mammalian Target of Rapamycin Activation.Brain Sci. 2020 Jun 30;10(7):409. doi: 10.3390/brainsci10070409. Brain Sci. 2020. PMID: 32629985 Free PMC article.
-
AKBA Promotes Axonal Regeneration via RhoA/Rictor to Repair Damaged Sciatic Nerve.Int J Mol Sci. 2022 Dec 14;23(24):15903. doi: 10.3390/ijms232415903. Int J Mol Sci. 2022. PMID: 36555556 Free PMC article.
-
Locally translated mTOR controls axonal local translation in nerve injury.Science. 2018 Mar 23;359(6382):1416-1421. doi: 10.1126/science.aan1053. Science. 2018. PMID: 29567716 Free PMC article.
-
Communicating pain: emerging axonal signaling in peripheral neuropathic pain.Front Neuroanat. 2024 Jul 9;18:1398400. doi: 10.3389/fnana.2024.1398400. eCollection 2024. Front Neuroanat. 2024. PMID: 39045347 Free PMC article. Review.
References
-
- Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F, Bader M, Blesch A, Pilpel Y, Twiss JL, Fainzilber M (2012) Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J 31:1350–1363. 10.1038/emboj.2011.494 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
