Host Range Restriction of Insect-Specific Flaviviruses Occurs at Several Levels of the Viral Life Cycle
- PMID: 28101536
- PMCID: PMC5227070
- DOI: 10.1128/mSphere.00375-16
Host Range Restriction of Insect-Specific Flaviviruses Occurs at Several Levels of the Viral Life Cycle
Abstract
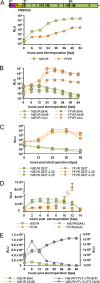
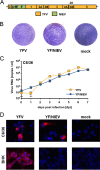
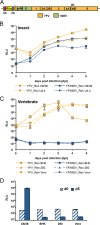
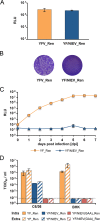
The genus Flavivirus contains emerging arthropod-borne viruses (arboviruses) infecting vertebrates, as well as insect-specific viruses (ISVs) (i.e., viruses whose host range is restricted to insects). ISVs are evolutionary precursors to arboviruses. Knowledge of the nature of the ISV infection block in vertebrates could identify functions necessary for the expansion of the host range toward vertebrates. Mapping of host restrictions by complementation of ISV and arbovirus genome functions could generate knowledge critical to predicting arbovirus emergence. Here we isolated a novel flavivirus, termed Niénokoué virus (NIEV), from mosquitoes sampled in Côte d'Ivoire. NIEV groups with insect-specific flaviviruses (ISFs) in phylogeny and grows in insect cells but not in vertebrate cells. We generated an infectious NIEV cDNA clone and a NIEV reporter replicon to study growth restrictions of NIEV in comparison to yellow fever virus (YFV), for which the same tools are available. Efficient RNA replication of the NIEV reporter replicon was observed in insect cells but not in vertebrate cells. Initial translation of the input replicon RNA in vertebrate cells was functional, but RNA replication did not occur. Chimeric YFV carrying the envelope proteins of NIEV was recovered via electroporation in C6/36 insect cells but did not infect vertebrate cells, indicating a block at the level of entry. Since the YF/NIEV chimera readily produced infectious particles in insect cells but not in vertebrate cells despite efficient RNA replication, restriction is also determined at the level of assembly/release. Taking the results together, the ability of ISF to infect vertebrates is blocked at several levels, including attachment/entry and RNA replication as well as assembly/release. IMPORTANCE Most viruses of the genus Flavivirus, e.g., YFV and dengue virus, are mosquito borne and transmitted to vertebrates during blood feeding of mosquitoes. Within the last decade, an increasing number of viruses with a host range exclusively restricted to insects in close relationship to the vertebrate-pathogenic flaviviruses were discovered in mosquitoes. To identify barriers that could block the arboviral vertebrate tropism, we set out to identify the steps at which the ISF replication cycle fails in vertebrates. Our studies revealed blocks at several levels, suggesting that flavivirus host range expansion from insects to vertebrates was a complex process that involved overcoming multiple barriers.
Keywords: host range restriction; infection barriers; insect-specific flavivirus.
Figures


Similar articles
-
Antigenic Characterization of New Lineage II Insect-Specific Flaviviruses in Australian Mosquitoes and Identification of Host Restriction Factors.mSphere. 2020 Jun 17;5(3):e00095-20. doi: 10.1128/mSphere.00095-20. mSphere. 2020. PMID: 32554715 Free PMC article.
-
Establishment and Application of Flavivirus Replicons.Adv Exp Med Biol. 2018;1062:165-173. doi: 10.1007/978-981-10-8727-1_12. Adv Exp Med Biol. 2018. PMID: 29845532 Review.
-
Chimeric viruses of the insect-specific flavivirus Palm Creek with structural proteins of vertebrate-infecting flaviviruses identify barriers to replication of insect-specific flaviviruses in vertebrate cells.J Gen Virol. 2019 Nov;100(11):1580-1586. doi: 10.1099/jgv.0.001326. J Gen Virol. 2019. PMID: 31524580
-
A New Clade of Insect-Specific Flaviviruses from Australian Anopheles Mosquitoes Displays Species-Specific Host Restriction.mSphere. 2017 Jul 12;2(4):e00262-17. doi: 10.1128/mSphere.00262-17. eCollection 2017 Jul-Aug. mSphere. 2017. PMID: 28713857 Free PMC article.
-
Unleashing Nature's Allies: Comparing the Vertical Transmission Dynamics of Insect-Specific and Vertebrate-Infecting Flaviviruses in Mosquitoes.Viruses. 2024 Sep 23;16(9):1499. doi: 10.3390/v16091499. Viruses. 2024. PMID: 39339975 Free PMC article. Review.
Cited by
-
Broad Host Tropism of Flaviviruses during the Entry Stage.Microbiol Spectr. 2023 Mar 21;11(2):e0528122. doi: 10.1128/spectrum.05281-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36943072 Free PMC article.
-
Agua Salud alphavirus defines a novel lineage of insect-specific alphaviruses discovered in the New World.J Gen Virol. 2020 Jan;101(1):96-104. doi: 10.1099/jgv.0.001344. J Gen Virol. 2020. PMID: 31674898 Free PMC article.
-
Different Degrees of 5'-to-3' DAR Interactions Modulate Zika Virus Genome Cyclization and Host-Specific Replication.J Virol. 2020 Feb 14;94(5):e01602-19. doi: 10.1128/JVI.01602-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31826997 Free PMC article.
-
Chimeric Vaccines Based on Novel Insect-Specific Flaviviruses.Vaccines (Basel). 2021 Oct 22;9(11):1230. doi: 10.3390/vaccines9111230. Vaccines (Basel). 2021. PMID: 34835160 Free PMC article. Review.
-
Development of Antibody Therapeutics against Flaviviruses.Int J Mol Sci. 2017 Dec 25;19(1):54. doi: 10.3390/ijms19010054. Int J Mol Sci. 2017. PMID: 29295568 Free PMC article. Review.
References
-
- Asgari S, Johnson KN. 2010. Insect virology. Caister Academic Press, Poole, UK.
LinkOut - more resources
Full Text Sources
Other Literature Sources

