Sequential Exposure of Bortezomib and Vorinostat is Synergistic in Multiple Myeloma Cells
- PMID: 28101809
- PMCID: PMC5826571
- DOI: 10.1007/s11095-017-2095-5
Sequential Exposure of Bortezomib and Vorinostat is Synergistic in Multiple Myeloma Cells
Abstract
Purpose: To examine the combination of bortezomib and vorinostat in multiple myeloma cells (U266) and xenografts, and to assess the nature of their potential interactions with semi-mechanistic pharmacodynamic models and biomarkers.
Methods: U266 proliferation was examined for a range of bortezomib and vorinostat exposure times and concentrations (alone and in combination). A non-competitive interaction model was used with interaction parameters that reflect the nature of drug interactions after simultaneous and sequential exposures. p21 and cleaved PARP were measured using immunoblotting to assess critical biomarker dynamics. For xenografts, data were extracted from literature and modeled with a PK/PD model with an interaction parameter.
Results: Estimated model parameters for simultaneous in vitro and xenograft treatments suggested additive drug effects. The sequence of bortezomib preincubation for 24 hours, followed by vorinostat for 24 hours, resulted in an estimated interaction term significantly less than 1, suggesting synergistic effects. p21 and cleaved PARP were also up-regulated the greatest in this sequence.
Conclusions: Semi-mechanistic pharmacodynamic modeling suggests synergistic pharmacodynamic interactions for the sequential administration of bortezomib followed by vorinostat. Increased p21 and cleaved PARP expression can potentially explain mechanisms of their enhanced effects, which require further PK/PD systems analysis to suggest an optimal dosing regimen.
Keywords: Bortezomib; pharmacodynamic modeling; synergistic combination; vorinostat.
Figures

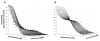
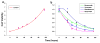


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

