Myeloid Cells in Asthma
- PMID: 28102118
- PMCID: PMC11687443
- DOI: 10.1128/microbiolspec.MCHD-0053-2016
Myeloid Cells in Asthma
Abstract
Asthma is a heterogeneous chronic inflammatory disorder of the airways, and not surprisingly, many myeloid cells play a crucial role in pathogenesis. Antigen-presenting dendritic cells are the first to recognize the allergens, pollutants, and viruses that are implicated in asthma pathogenesis, and subsequently initiate the adaptive immune response by migrating to lymph nodes. Eosinophils are the hallmark of type 2 inflammation, releasing toxic compounds in the airways and contributing to airway remodeling. Mast cells and basophils control both the early- and late-phase allergic response and contribute to alterations in smooth muscle reactivity. Finally, relatively little is known about neutrophils and macrophages in this disease. Although many of these myeloid cells respond well to treatment with inhaled steroids, there is now an increasing armamentarium of targeted biologicals that can specifically eliminate only one myeloid cell population, like eosinophils. It is only with those new tools that we will be able to fully understand the role of myeloid cells in chronic asthma in humans.
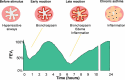
Figures

References
-
- Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, Wenzel SE, Peters SP, Meyers DA, Bleecker ER. 2014. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol 133:1557–1563.e5. 10.1016/j.jaci.2013.10.011. [PubMed] - DOI - PMC - PubMed
-
- Wenzel SE. 2012. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 18:716–725. - PubMed
-
- Anderson GP. 2008. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 372:1107–1119. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

